Abstract
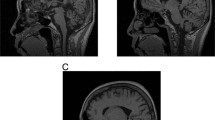
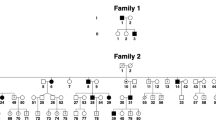
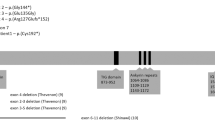
The gene for spinocerebellar ataxia 7 (SCA7) has been mapped to chromosome 3p12–13. By positional cloning, we have identified a new gene of unknown function containing a CAG repeat that is expanded in SCA7 patients. On mutated alleles, CAG repeat size is highly variable, ranging from 38 to 130 repeats, whereas on normal alleles it ranges from 7 to 17 repeats. Gonadal instability in SCA7 is greater than that observed in any of the seven known neuro-degenerative diseases caused by translated CAG repeat expansions, and is markedly associated with paternal transmissions. SCA7 is the first such disorder in which the degenerative process also affects the retina.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Konigsmark, B.W. & Weiner, L.P. The olivopontocerebellar atrophies: a review. Medicine (Baltimore) 49, 227–241 (1970).
Berciano, J. Olivopontocerebellar atrophy: a review of 117 cases. J. Neurol. Sci. 53, 253–272 (1982).
Harding, A.E. The clinical features and classification of the late onset autosomal dominant cerebellar ataxias: a study of 11 families, including descendants of the ‘the Drew family of Walworth’. Brain 105, 1–28 (1982).
Harding, A.E. Clinical features and classification of inherited ataxias. Adv. Neurol. 61, 1–14 (1993).
Enevoldson, T.P., Sanders, M.D. & Harding, A.E. Autosomal dominant cerebellar ataxia with pigmentary macular dystrophy: a clinical and genetic study of eight families. Brain 117, 445–460 (1994).
Benomar, A. et al. Autosomal-dominant cerebellar ataxia with retinal degeneration (ADCA type II) is genetically different from ADCA type I. Ann. Neurol. 35, 439–444 (1994).
Benomar, A. et al. The gene for autosomal dominant cerebellar ataxia with pigmentary macular dystrophy maps to chromosome 3p12–p21.1. Nature Genet. 10, 84–88 (1995).
David, G. et al. The gene for autosomal dominant cerebellar ataxia type II is located in a 5-cM region in 3p12-p13: genetic and physical mapping of the SCA7 locus. Am. J. Hum. Genet. 59, 1328–1336 (1996).
Gouw, L.G. et al. Retinal degeneration characterizes a spinocerebellar ataxia mapping to chromosome 3p. Nature Genet. 10, 89–93 (1995).
Holmberg, M. et al. Localization of autosomal dominant cerebellar ataxia associated with retinal degeneration and anticipation to chromosome 3p12–p21.1. Hum. Mol. Genet. 4, 1441–1445 (1995).
Krols, L. et al. Refinement of the locus for autosomal dominant cerebellar ataxia type II to chromosome 3p21.1–14.1. Hum. Genet. 99, 225–232 (1997).
Jöbsis, G.J. et al. Autosomal dominant cerebellar ataxia with retinal degeneration (ADCA II): clinical and neuropathological findings in two pedigrees and genetic linkage to 3p12–p21.1. J. Neurol. Neurosurg. Psychiatry 367–371 (1997).
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Orr, H.T. et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nature Genet. 4, 221–226 (1993).
Pulst, S.-M. et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nature Genet. 14, 269–276 (1996).
Sanpei, K. et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nature Genet. 14, 277–284 (1996).
Imbert, G. et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nature Genet. 14, 285–291 (19%).
Kawaguchi, Y. et al. CAG expansion in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nature Genet. 8, 221–227 (1994).
Koide, R. et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nature Genet. 6, 9–13 (1994).
Nagafuchi, S. et al. Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nature Genet. 8, 177–182 (1994).
Trottier, Y. et al. Polyglutamine expansion as a pathological epitope in Huntington's disease and four dominant cerebellar ataxias. Nature 378, 403–406 (1995).
Stevanin, G. et al. Screening for proteins with polyglutamine expansions in autosomal dominant cerebellar ataxias. Hum. Mol. Genet. 5, 1887–1892 (1996).
Lindblad, K. et al. An expanded CAG repeat sequence in spinocerebellar ataxia type 7. Genome Res. 6, 965–971 (1996).
Uberbacher, E.C. & Mural, R.J. Locating protein-coding regions in human DNA sequences by a multiple sensor-neural network approach. Proc. Natl. Acad. Sci. USA 88, 11261–11265 (1991).
Cancel, G. et al. Marked phenotypic heterogeneity associated with expansion of a CAG repeat sequence at the spinocerebellar ataxia 3/Machado-Joseph disease locus. Am. J. Hum. Genet. 57, 809–816 (1995).
Cancel, G. et al. Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum. Mol. Genet. 6, 709–715 (1997).
Kozak, M. An analysis of 5-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic. Acids. Res. 15, 8125–8148 (1987).
Dingwall, C. & Laskey, R.A. Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 16, 478–481 (1991).
Mandel, J.-L. Breaking the rule of three. Nature 386, 767–769 (1997).
Zhuchenko, O. et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the a1A-voltage-dependent calcium channel. Nature Genet 15, 62–69 (1997).
Diirr, A. & Brice, A. Genetics of movement disorders. Curr. Opin. Neurol. 9, 290–297 (1996).
Martin, J.J. et al. On an autosomal dominant form of retinal-cerebellar degeneration: an autopsy study of five patients in one family. Acta Neuropathol. 88, 277–286 (1994).
Ikeuchi, T. et al. Dentatorubral-pallidoluysian atrophy (DRPLA): close correlation of CAG repeat expansions with the wide spectrum of clinical presentations and prominent anticipation. Semin. Cell Biol. 6, 37–44 (1995).
Leeflang, E.P. et al. Single sperm analysis of the trinucleotide repeats in the Huntington's disease gene: quantification of the mutation frequency spectrum. Hum. Mol. Genet. 4, 1519–1526 (1995).
Chong, S.S. et al. Contribution of DNA sequence and CAG size to mutation frequencies of intermediate alleles for huntington disease: evidence from single sperm analyses. Hum. Mol. Genet. 6, 301–309 (1997).
Igarashi, S. et al. Intergenerational instability of the CAG repeat of the gene for Machado–Joseph disease (MJD1) is affected by the genotype of the normal chromosome: implications for the molecular mechanisms of the instability of the CAG repeat. Hum. Mol. Genet. 5, 923–932 (1996).
Takiyama, Y. et al. Single sperm analysis of the CAG repeats in the gene for Machado–Joseph disease (MJD1): evidence for non-Mendelian transmission of the MJD1 gene and for the effect of the intragenic CGG/GGG polymorphism on the intergenerational instability. Hum. Mol. Genet. 6, 1063–1068 (1997).
Kang, S., Jaworski, A., Ohshima, K. & Wells, R.D. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E coil. Nature Genet. 10, 213–218 (1995).
Gerber, H.P. et al. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science 263, 808–811 (1994).
Ross, C.A. When more is less: pathogenesis of glutamine repeat neurodegenerative disorders. Neuron 15, 493–496 (1995).
Stevanin, G. et al. Linkage disequilibrium between the spinocerebellar ataxia 3/ Machado-Joseph disease mutation and two intragenic polymorphisms, one of which, X359Y, affects the stop codon. Am. J. Hum. Genet. 60, 1548–1552 (1997).
Church, G.M. & Gilbert, W. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81, 1991–1995 (1984).
Maniatis, T., Fritsch, E.F. & Sambrook, J. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989).
Ruberg, M. et al. Is differential regulation of mitochondrial transcripts in Parkinson's disease related to apoptosis? J. Neurochem. 2098–2110 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
David, G., Abbas, N., Stevanin, G. et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet 17, 65–70 (1997). https://doi.org/10.1038/ng0997-65
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0997-65
This article is cited by
-
Clinical and genetic spectrums of 413 North African families with inherited retinal dystrophies and optic neuropathies
Orphanet Journal of Rare Diseases (2022)
-
Abnormal molecular signatures of inflammation, energy metabolism, and vesicle biology in human Huntington disease peripheral tissues
Genome Biology (2022)
-
Polyglutamine-expanded ATXN7 alters a specific epigenetic signature underlying photoreceptor identity gene expression in SCA7 mouse retinopathy
Journal of Biomedical Science (2022)
-
The length of uninterrupted CAG repeats in stem regions of repeat disease associated hairpins determines the amount of short CAG oligonucleotides that are toxic to cells through RNA interference
Cell Death & Disease (2022)
-
Rapid and comprehensive diagnostic method for repeat expansion diseases using nanopore sequencing
npj Genomic Medicine (2022)