Abstract
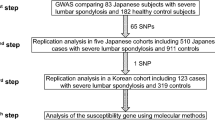
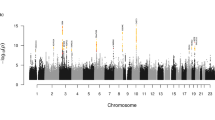
Lumbar disc disease (LDD) is caused by degeneration of intervertebral discs of the lumbar spine. One of the most common musculoskeletal disorders1, LDD has strong genetic determinants2,3,4. Using a case-control association study, we identified a functional SNP (1184T → C, resulting in the amino acid substitution I395T) in CILP, which encodes the cartilage intermediate layer protein, that acts as a modulator of LDD susceptibility. CILP was expressed abundantly in intervertebral discs, and its expression increased as disc degeneration progressed. CILP colocalized with TGF-β1 in clustering chondrocytes and their territorial matrices in intervertebral discs. CILP inhibited TGF-β1–mediated induction of cartilage matrix genes through direct interaction with TGF-β1 and inhibition of TGF-β1 signaling. The susceptibility-associated 1184C allele showed increased binding and inhibition of TGF-β1. Therefore, we conclude that the extracellular matrix protein CILP regulates TGF-β signaling and that this regulation has a crucial role in the etiology and pathogenesis of LDD. Our study also adds to the list of connective tissue diseases that are associated with TGF-β.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 354, 581–585 (1999).
Matsui, H. et al. Familial predisposition for lumbar degenerative disc disease. A case-control study. Spine 23, 1029–1034 (1998).
Battie, M.C. et al. Similarities in degenerative findings on magnetic resonance images of the lumbar spines of identical twins. J. Bone Joint Surg. Am. 77, 1662–1670 (1995).
Sambrook, P.N., MacGregor, A.J. & Spector, T.D. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 42, 366–372 (1999).
Saal, J.A. & Saal, J.S. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine 14, 431–437 (1989).
Annunen, S. et al. An allele of COL9A2 associated with intervertebral disc disease. Science 285, 409–412 (1999).
Kimura, T. et al. Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int. Orthop. 20, 177–181 (1996).
Watanabe, H., Nakata, K., Kimata, K., Nakanishi, I. & Yamada, Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc. Natl. Acad. Sci. USA 94, 6943–6947 (1997).
Kawaguchi, Y. et al. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine 24, 2456–2460 (1999).
Freedman, M.L. et al. Assessing the impact of population stratification on genetic association studies. Nat. Genet. 36, 388–393 (2004).
Johnson, K., Farley, D., Hu, S.I. & Terkeltaub, R. One of two chondrocyte-expressed isoforms of cartilage intermediate-layer protein functions as an insulin-like growth factor 1 antagonist. Arthritis Rheum. 48, 1302–1314 (2003).
Neptune, E.R. et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 33, 407–411 (2003).
Kizawa, H. et al. An aspartic acid repeat polymorphism in asporin negatively affects chondrogenesis and increases susceptibility to osteoarthritis. Nat. Genet. 37, 138–144 (2005).
Pattison, S.T., Melrose, J., Ghosh, P. & Taylor, T.K. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by Transforming Growth Factor-β1 and insulin like growth factor-I. Cell. Biol. Int. 25, 679–689 (2001).
Baffi, M.O. et al. Conditional deletion of the TGF-β type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev. Biol. 276, 124–142 (2004).
Lorenzo, P., Neame, P., Sommarin, Y. & Heinegard, D. Cloning and deduced amino acid sequence of a novel cartilage protein (CILP) identifies a proform including a nucleotide pyrophosphohydrolase. J. Biol. Chem. 273, 23469–23475 (1998).
Watanabe, H., Caestecker, M.P. & Yamada, Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem. 276, 14466–14473 (2001).
Young, G.D. & Murphy-Ullrich, J.E. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-β complex. J. Biol. Chem. 279, 47633–47642 (2004).
Chen, J., Yan, W. & Setton, L.A. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 24, 573–583 (2004).
Border, W.A. et al. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 360, 361–364 (1992).
Isaka, Y. et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat. Med. 2, 418–423 (1996).
Tolonen, J. et al. Transforming growth factor beta receptor induction in herniated intervertebral disc tissue: an immunohistochemical study. Eur. Spine J. 10, 172–176 (2001).
Serra, R. et al. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J. Cell Biol. 139, 541–552 (1997).
Yang, X. et al. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 153, 35–46 (2001).
Mizuguchi, T. et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 36, 855–860 (2004).
Kinoshita, A. et al. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat. Genet. 26, 19–20 (2000).
Schneiderman, G. et al. Magnetic resonance imaging in the diagnosis of disc degeneration: correlation with discography. Spine 12, 276–281 (1987).
Chiba, K., Andersson, G.B., Masuda, K. & Thonar, E.J. Metabolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine 22, 2885–2893 (1997).
Masuda, I., Hamada, J., Haas, A.L., Ryan, L.M. & McCarthy, D.J. A unique ectonucleotide pyrophosphohydrolase associated with porcine chondrocyte-derived vesicles. J. Clin. Invest. 95, 699–704 (1995).
Acknowledgements
We thank the affected individuals for participating in this study; H. Hirabayashi, T. Kono, N. Hosogane, H. Hase, T. Ogura, T. Tanaka, M. Sasahara, A. Sano, H. Ishihara, M. Kanamori and K. Toyoshima for assistance; and Y. Takanashi and T. Matsushima for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Real-time RT-PCR analysis of CILP mRNA in various human tissues and cell lines. (PDF 86 kb)
Supplementary Fig. 2
CILP expression in intervertebral disc tissue from LDD patients. (PDF 62 kb)
Supplementary Fig. 3
CILP expression in intervertebral disc tissues from a normal subject and an LDD patient (Schneiderman's grade 3). (PDF 61 kb)
Supplementary Fig. 4
Lack of NTPPHase activity in COS-7 cells expressing F-CILP. (PDF 75 kb)
Supplementary Fig. 5
Purified F-CILP containing p.I395 or p.T395, visualized by silver staining. (PDF 75 kb)
Supplementary Fig. 6
Effects of the LDD-associated SNP (p.I395T) in N-CILP on TGF-β1-induced expression of the aggrecan and type II collagen genes in rabbit nucleus pulposus cells. (PDF 94 kb)
Supplementary Table 1
Assessment of population stratification. (PDF 61 kb)
Supplementary Table 2
Haplotype analysis of CILP with lumbar disc herniation. (PDF 96 kb)
Rights and permissions
About this article
Cite this article
Seki, S., Kawaguchi, Y., Chiba, K. et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet 37, 607–612 (2005). https://doi.org/10.1038/ng1557
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1557
This article is cited by
-
Can cartilage intermediate layer protein 1 (CILP1) use as a novel biomarker for canine myxomatous mitral valve degeneration levels or not?
BMC Veterinary Research (2023)
-
A novel biomarker of MMP-cleaved cartilage intermediate layer protein-1 is elevated in patients with rheumatoid arthritis, ankylosing spondylitis and osteoarthritis
Scientific Reports (2023)
-
Expression of CILP-2 and DDR2 and ultrastructural changes in the articular cartilage of patients with knee osteoarthritis undergoing total knee arthroplasty: a pilot morphological study
Medical Molecular Morphology (2023)
-
Intervertebral disc degeneration in mice with type II diabetes induced by leptin receptor deficiency
BMC Musculoskeletal Disorders (2020)
-
CHRNA5/CHRNA3 gene cluster is a risk factor for lumbar disc herniation: a case-control study
Journal of Orthopaedic Surgery and Research (2019)