Abstract
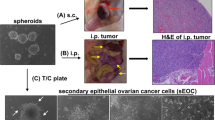
We have found that EEF1A2, the gene encoding protein elongation factor EEF1A2 (also known as eEF-1α2), is amplified in 25% of primary ovarian tumors and is highly expressed in approximately 30% of ovarian tumors and established cell lines. We have also demonstrated that EEF1A2 has oncogenic properties: it enhances focus formation, allows anchorage-independent growth and decreases the doubling time of rodent fibroblasts. In addition, EEF1A2 expression made NIH3T3 fibroblasts tumorigenic and increased the growth rate of ES-2 ovarian carcinoma cells xenografted in nude mice. Thus, EEF1A2 and the process of protein elongation are likely to be critical in the development of ovarian cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Courjal, F. et al. DNA amplifications at 20q13 and MDM2 define distinct subsets of evolved breast and ovarian tumours. Br. J. Cancer 74, 1984–1989 (1996).
Sonoda, G. et al. Comparative genomic hybridization detects frequent overrepresentation of chromosomal material from 3q26, 8q24, and 20q13 in human ovarian carcinomas. Genes Chromosomes Cancer 20, 320–328 (1997).
Diebold, J. et al. 20q13 and cyclin D1 in ovarian carcinomas. Analysis by fluorescence in situ hybridization. J. Pathol. 190, 564–571 (2000).
Lund, A., Knudsen, S.M., Vissing, H., Clark, B. & Tommerup, N. Assignment of human elongation factor 1α genes: EEF1A maps to chromosome 6q14 and EEF1A2 to 20q13. 3. Genomics 36, 359–361 (1996).
Hershey, J.W. Translational control in mammalian cells. Annu. Rev. Biochem. 60, 717–755 (1991).
Condeelis, J. Elongation factor 1 α, translation and the cytoskeleton. Trends Biochem. Sci. 20, 169–170 (1995).
Yang, F., Demma, M., Warren, V., Dharmawardhane, S. & Condeelis, J. Identification of an actin-binding protein from Dictyostelium as elongation factor 1α. Nature 347, 494–496 (1990).
Shiina, N., Gotoh, Y., Kubomura, N., Iwamatsu, A. & Nishida, E. Microtubule severing by elongation factor 1 α. Science 266, 282–285 (1994).
Lee, S., Francoeur, A.M., Liu, S. & Wang, E. Tissue-specific expression in mammalian brain, heart, and muscle of S1, a member of the elongation factor-1 α gene family. J. Biol. Chem. 267, 24064–24068 (1992).
Knudsen, S.M., Frydenberg, J., Clark, B.F. & Leffers, H. Tissue-dependent variation in the expression of elongation factor-1 α isoforms: isolation and characterisation of a cDNA encoding a novel variant of human elongation-factor 1 α. Eur. J. Biochem. 215, 549–554 (1993).
Berry, R. et al. Evidence for a prostate cancer-susceptibility locus on chromosome 20. Am. J. Hum. Genet. 67, 82–91 (2000).
Kjaer, S. et al. Generation and epitope mapping of high-affinity scFv to eukaryotic elongation factor 1A by dual application of phage display. Eur. J. Biochem. 268, 3407–3415 (2001).
Land, H., Parada, L.F. & Weinberg, R.A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304, 596–602 (1983).
Provencher, D.M. et al. Characterization of four novel epithelial ovarian cancer cell lines. In Vitro Cell. Dev. Biol. Anim. 36, 357–361 (2000).
Collins, C. et al. Positional cloning of ZNF217 and NABC1: genes amplified at 20q13.2 and overexpressed in breast carcinoma. Proc. Natl Acad. Sci. USA 95, 8703–8708 (1998).
Albertson, D.G. et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nature Genet. 25, 144–146 (2000).
Bischoff, J.R. et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3052–3065 (1998).
Nonet, G.H. et al. The ZNF217 gene amplified in breast cancers promotes immortalization of human mammary epithelial cells. Cancer Res. 61, 1250–1254 (2001).
Walters, M.R. Newly identified actions of the vitamin D endocrine system. Endocr. Rev. 13, 719–764 (1992).
Shultz, L.D., Sweet, H.O., Davisson, M.T. & Coman, D.R. 'Wasted', a new mutant of the mouse with abnormalities characteristic to ataxia telangiectasia. Nature 297, 402–404 (1982).
Chambers, D.M., Peters, J. & Abbott, C.M. The lethal mutation of the mouse wasted (wst) is a deletion that abolishes expression of a tissue-specific isoform of translation elongation factor 1α, encoded by the Eef1a2 gene. Proc. Natl Acad. Sci. USA 95, 4463–4468 (1998).
Potter, M., Bernstein, A. & Lee, J.M. The wst gene regulates multiple forms of thymocyte apoptosis. Cell. Immunol. 188, 111–117 (1998).
Kerekatte, V. et al. The proto-oncogene/translation factor eIF4E: a survey of its expression in breast carcinomas. Int. J. Cancer 64, 27–31 (1995).
Anthony, B., Carter, P. & De Benedetti, A. Overexpression of the proto-oncogene/translation factor 4E in breast-carcinoma cell lines. Int. J. Cancer 65, 858–863 (1996).
Sonenberg, N. Translation factors as effectors of cell growth and tumorigenesis. Curr. Opin. Cell Biol. 5, 955–960 (1993).
Shen, R., Su, Z.Z., Olsson, C.A. & Fisher, P.B. Identification of the human prostatic carcinoma oncogene PTI-1 by rapid expression cloning and differential RNA display. Proc. Natl Acad. Sci. USA 92, 6778–6782 (1995).
Demetrick, D.J. The use of archival frozen tumor tissue imprint specimens for fluorescence in situ hybridization. Mod. Pathol. 9, 133–136 (1996).
Acknowledgements
We thank A. Al Abadi, R. Austin, J. Hanlon, S. Innocente, S. Lhotak, S. Popovic and E. Seidlitz for help with many of the assays and A. Bernstein, H. Ghosh, J. Hassell, H. Hirte, B. Muller, M. Rozakis-Adcock, G. Singh and P. Whyte for discussion and critical reading of the manuscript. We thank K. Dougherty and H. Blackborrow for secretarial assistance. We acknowledge the sharing of cell lines and information by P. Tonin, T. Hudson, D. Provencher and A.-M. Mes-Masson. This work was supported by funding from the National Cancer Institute of Canada and the Hamilton Regional Cancer Centre Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Anand, N., Murthy, S., Amann, G. et al. Protein elongation factor EEF1A2 is a putative oncogene in ovarian cancer. Nat Genet 31, 301–305 (2002). https://doi.org/10.1038/ng904
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng904
This article is cited by
-
Oncogenic activation of EEF1A2 expression: a journey from a putative to an established oncogene
Cellular & Molecular Biology Letters (2024)
-
EEF1A2 promotes HIF1A mediated breast cancer angiogenesis in normoxia and participates in a positive feedback loop with HIF1A in hypoxia
British Journal of Cancer (2024)
-
Immunoproteasome-specific subunit PSMB9 induction is required to regulate cellular proteostasis upon mitochondrial dysfunction
Nature Communications (2023)
-
Initiation and elongation factor co-expression correlates with recurrence and survival in epithelial ovarian cancer
Journal of Ovarian Research (2022)
-
Prognostic value and immune relevancy of a combined autophagy-, apoptosis- and necrosis-related gene signature in glioblastoma
BMC Cancer (2022)