Abstract
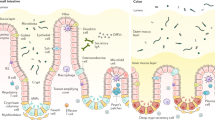
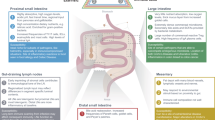
Frequent microbial and nonmicrobial challenges to epithelial cells trigger discrete pathways, promoting molecular changes such as the secretion of specific cytokines and chemokines and alterations to molecules displayed at the epithelial cell surface. In combination, these molecules impose key decisions on innate and adaptive immune cells. Depending on context, those decisions can be as diverse as those imposed by professional antigen-presenting cells, benefiting the host by balancing immune competence with the avoidance of immunopathology. Nonetheless, this potency of epithelial cells is also consistent with the causal contribution of epithelial dysregulation to myriad inflammatory diseases. This pathogenic axis provides an attractive target for tissue-specific clinical manipulation. In this context, a research goal should be to identify all molecules used by epithelial cells to instruct immune cells. We term this the 'epimmunome'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Anderson, G. & Jenkinson, E.J. Lymphostromal interactions in thymic development and function. Nat. Rev. Immunol. 1, 31–40 (2001).
Hayday, A., Theodoridis, E., Ramsburg, E. & Shires, J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2, 997–1003 (2001).
Kronenberg, M. & Havran, W.L. Frontline T cells: γδ T cells and intraepithelial lymphocytes. Immunol. Rev. 215, 5–7 (2007).
Laky, K. et al. Enterocyte expression of interleukin 7 induces development of γδ T cells and Peyer's patches. J. Exp. Med. 191, 1569–1580 (2000).
Lai, Y.-G. IL-15 promotes survival but not effector function differentiation of CD8+ TCRαβ+ intestinal intraepithelial lymphocytes. J. Immunol. 163, 5843–5850 (1999).
Eyerich, S. et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119, 3573–3585 (2009).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Thorey, I.S. et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J. Biol. Chem. 276, 35818–35825 (2001).
Ryckman, C., Vandal, K., Rouleau, P., Talbot, M. & Tessier, P.A. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 170, 3233–3242 (2003).
Ghavami, S. et al. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur. J. Pharmacol. 625, 73–83 (2009).
Bando, M. et al. Interleukin-1α regulates antimicrobial peptide expression in human keratinocytes. Immunol. Cell Biol. 85, 532–537 (2007).
Carroll, J.M., Romero, M.R. & Watt, F.M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83, 957–968 (1995).
Scaffidi, P., Misteli, T. & Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Sims, G.P., Rowe, D.C., Rietdijk, S.T., Herbst, R. & Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 (2010).
Kainulainen, V., Wang, H., Schick, C. & Bernfield, M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J. Biol. Chem. 273, 11563–11569 (1998).
Bruynzeel, I., Koopman, G., van der Raaij, L.M., Pals, S.T. & Willemze, R. CD44 antibody stimulates adhesion of peripheral blood T cells to keratinocytes through the leukocyte function-associated antigen-1/intercellular adhesion molecule-1 pathway. J. Invest. Dermatol. 100, 424–428 (1993).
Kaser, A. & Blumberg, R.S. Endoplasmic reticulum stress in the intestinal epithelium and inflammatory bowel disease. Semin. Immunol. 21, 156–163 (2009).
Bertolotti, A. et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1β-deficient mice. J. Clin. Invest. 107, 585–593 (2001).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Kaser, A., Zeissig, S. & Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Heazlewood, C.K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Kühn, R., Löhler, J., Rennick, D., Rajewsky, K. & Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Glocker, E.O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Shkoda, A. et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 132, 190–207 (2007).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Luedde, T. et al. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11, 119–132 (2007).
Pasparakis, M. et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866 (2002).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Ziegler, S.F. & Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 11, 289–293 (2010).
Tang, H. et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat. Immunol. 11, 608–617 (2010).
Kouzaki, H., O'Grady, S.M., Lawrence, C.B. & Kita, H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J. Immunol. 183, 1427–1434 (2009).
Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10, 131–144 (2010).
Slack, E. et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325, 617–620 (2009).
Gong, J. et al. Epithelial-specific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin. Immunol. published online, doi:10.1016/j.clim.2010.04.001 (10 May 2010).
Fritz, J., Le Bourhis, L., Magalhaes, J. & Philpott, D. Innate immune recognition at the epithelial barrier drives adaptive immunity: APCs take the back seat. Trends Immunol. 29, 41–49 (2008).
Fritz, J.H. et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 26, 445–459 (2007).
Kobayashi, K.S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Magalhaes, J.G. et al. Nod2-dependent Th2 polarization of antigen-specific immunity. J. Immunol. 181, 7925–7935 (2008).
Travassos, L.H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62 (2010).
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009).
Lawrence, T., Bebien, M., Liu, G.Y., Nizet, V. & Karin, M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434, 1138–1143 (2005).
Owyang, A.M. et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 (2006).
Angkasekwinai, P. et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204, 1509–1517 (2007).
Saenz, S.A., Taylor, B.C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008).
Al-Shami, A., Spolski, R., Kelly, J., Keane-Myers, A. & Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202, 829–839 (2005).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002).
Yoo, J. et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 202, 541–549 (2005).
Shires, J., Theodoridis, E. & Hayday, A.C. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15, 419–434 (2001).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Pastorelli, L. et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc. Natl. Acad. Sci. USA 107, 8017–8022 (2010).
Perrigoue, J.G., Marshall, F.A. & Artis, D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell. Microbiol. 10, 1757–1764 (2008).
Zaph, C. et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 205, 2191–2198 (2008).
Lajoie-Kadoch, S. et al. TNF-α and IFN-γ inversely modulate expression of the IL-17E receptor in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L1238–L1246 (2006).
Hanabuchi, S. et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J. Immunol. 184, 2999–3007 (2010).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 (2005).
Li, M. et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 103, 11736–11741 (2006).
Iliev, I.D. et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58, 1481–1489 (2009).
Maynard, C.L. et al. Contrasting roles for all-trans retinoic acid in TGF-beta-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med. 206, 343–357 (2009).
Kato, A. & Schleimer, R.P. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 19, 711–720 (2007).
Niess, J.H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Strid, J., Tigelaar, R.E. & Hayday, A.C. Skin immune surveillance by T cells–a new order? Semin. Immunol. 21, 110–120 (2009).
Dinarello, C.A. The biological properties of interleukin-1. Eur. Cytokine Netw. 5, 517–531 (1994).
Jerde, T.J. & Bushman, W. IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Science Signal. 2, ra49 (2009).
Groves, R.W., Mizutani, H., Kieffer, J.D. & Kupper, T.S. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc. Natl. Acad. Sci. USA 92, 11874–11878 (1995).
Renne, J., Schafer, V., Werfel, T. & Wittmann, M. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. Br. J. Dermatol. 162, 1198–1205 (2010).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Watanabe, H. et al. Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J. Invest. Dermatol. 127, 1956–1963 (2007).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Zaki, M. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Takagi, H. et al. Contrasting action of IL-12 and Il-18 in the development of dextran sulphate colitis in mice. Scand. J. Immunol. 38, 837–844 (2003).
Blumberg, H. et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 204, 2603–2614 (2007).
Lee, H.C. & Ziegler, S.F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci. USA 104, 914–919 (2007).
Netea, M.G. et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1β and IL-6 production through a caspase 1-dependent mechanism. Proc. Natl. Acad. Sci. USA 102, 16309–16314 (2005).
Shioya, M. et al. Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clin. Exp. Immunol. 149, 480–486 (2007).
Calabrese, F. et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178, 894–901 (2008).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Fagarasan, S., Kawamoto, S., Kanagawa, O. & Suzuki, K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 28, 243–273 (2010).
Xu, W. et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8, 294–303 (2007).
Schleimer, R.P., Kato, A., Kern, R., Kuperman, D. & Avila, P.C. Epithelium: at the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 120, 1279–1284 (2007).
Schluns, K.S. et al. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc. Natl. Acad. Sci. USA 101, 5616–5621 (2004).
Yu, Q. et al. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J. Immunol. 176, 6180–6185 (2006).
Muehlhoefer, A. et al. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J. Immunol. 164, 3368–3376 (2000).
Sharp, L.L., Jameson, J.M., Cauvi, G. & Havran, W.L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6, 73–79 (2005).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Nava, P. et al. Interferon-γ regulates intestinal epithelial homeostasis through converging β-catenin signaling pathways. Immunity. 32, 392–402 (2010).
Wang, Y. et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune response against mucosal bacterial infection. Immunity. 32, 403–413 (2010).
Sharpe, A.H. Mechanisms of costimulation. Immunol. Rev. 229, 5–11 (2009).
Kim, J. et al. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 33, 280–289 (2005).
Arnett, H.A. et al. BTNL2, a butyrophilin/B7-like molecule, is a negative costimulatory molecule modulated in intestinal inflammation. J. Immunol. 178, 1523–1533 (2007).
Nguyen, T., Liu, X.K., Zhang, Y. & Dong, C. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J. Immunol. 176, 7354–7360 (2006).
Arnett, H.A., Escobar, S.S. & Viney, J.L. Regulation of costimulation in the era of butyrophilins. Cytokine 46, 370–375 (2009).
Lian, Y., Yue, J., Han, M., Liu, J. & Liu, L. Analysis of the association between BTNL2 polymorphism and tuberculosis in Chinese Han population. Infect. Genet. Evol. 10, 517–521 (2010).
Mochida, A. et al. Butyrophilin-like 2 gene is associated with ulcerative colitis in the Japanese under strong linkage disequilibrium with HLA-DRB1*1502. Tissue Antigens 70, 128–135 (2007).
Spagnolo, P. et al. Analysis of BTNL2 genetic polymorphisms in British and Dutch patients with sarcoidosis. Tissue Antigens 70, 219–227 (2007).
Yang, Y. et al. Characterization of B7S3 as a novel negative regulator of T cells. J. Immunol. 178, 3661–3667 (2007).
Witherden, D.A. et al. Novel functional roles for JAML and CAR in epithelial γδ T cell activation. Science (in the press).
Verdino, P., Witherden, D.A., Havran, W.L. & Wilson, I.A. Molecular interaction of CAR and JAML induces recruitment of the central cell signal transducer PI3K. Science (in the press).
Eagle, R.A. & Trowsdale, J. Promiscuity and the single receptor: NKG2D. Nat. Rev. Immunol. 7, 737–744 (2007).
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Groh, V. et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 96, 6879–6884 (1999).
Strid, J. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 9, 146–154 (2008).
Whang, M.I., Guerra, N. & Raulet, D.H. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J. Immunol. 182, 4557–4564 (2009).
Hershberg, R.M. & Mayer, L.F. Antigen processing and presentation by intestinal epithelial cells—polarity and complexity. Immunol. Today 21, 123–128 (2000).
Hayday, A.C. γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Meresse, B. et al. Coordinated induction by IL-15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Hue, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–177 (2004).
Fahrer, A.M. et al. Attributes of gamma delta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc. Natl. Acad. Sci. USA 98, 10261–10266 (2001).
Jameson, J. et al. A role for skin γδ T cells in wound repair. Science 296, 747–749 (2002).
Bryceson, Y.T., Ljunggren, H.G. & Long, E.O. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 114, 2657–2666 (2009).
Cepek, K.L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372, 190–193 (1994).
Daniels, M.A. et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 (2006).
Lewis, J.M. et al. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 7, 843–850 (2006).
Boyden, L.M. et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 40, 656–662 (2008).
Acknowledgements
We thank many colleagues for insight. Supported by the Wellcome Trust and an UK Medical Research Council program grant for psoriasis research (A.H.); the European Molecular Biology Organization and the Marie Curie Program (M.S.), and the US National Institutes of Health (C.J., W.H.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Swamy, M., Jamora, C., Havran, W. et al. Epithelial decision makers: in search of the 'epimmunome'. Nat Immunol 11, 656–665 (2010). https://doi.org/10.1038/ni.1905
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1905
This article is cited by
-
Bojungikgi-tang improves skin barrier function and immune response in atopic dermatitis mice fed a low aryl hydrocarbon receptor ligand diet
Chinese Medicine (2023)
-
Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice
Scientific Reports (2020)
-
c-Rel orchestrates energy-dependent epithelial and macrophage reprogramming in fibrosis
Nature Metabolism (2020)
-
Personalisierte Medizin in der Allergologie
Der Hautarzt (2019)
-
Microarray analysis of human keratinocytes from different anatomic sites reveals site-specific immune signaling and responses to human papillomavirus type 16 transfection
Molecular Medicine (2018)