Abstract
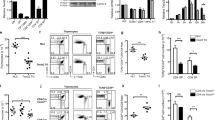
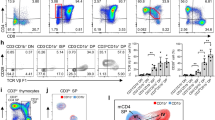
Signaling via the T cell antigen receptor (TCR) during the CD4+CD8+ double-positive developmental stage determines thymocyte selection and lineage commitment. Here we describe a previously uncharacterized T cell–expressed protein, Tespa1, with critical functions during the positive selection of thymocytes. Tespa1−/− mice had fewer mature thymic CD4+ and CD8+ T cells, which reflected impaired thymocyte development. Tespa1 associated with the TCR signaling components PLC-γ1 and Grb2, and Tespa1 deficiency resulted in attenuated TCR signaling, as reflected by defective activation of the Erk–AP-1 and Ca2+-NFAT pathways. Our findings demonstrate that Tespa1 is a component of the TCR signalosome and is essential for T cell selection and maturation through the regulation of TCR signaling during T cell development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Starr, T.K., Jameson, S.C. & Hogquist, K.A. Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003).
Germain, R.N. T-cell development and the CD4–CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322 (2002).
Bosselut, R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat. Rev. Immunol. 4, 529–540 (2004).
Singer, A., Adoro, S. & Park, J.H. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 8, 788–801 (2008).
Werlen, G. & Palmer, E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr. Opin. Immunol. 14, 299–305 (2002).
Brockmeyer, C. et al. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J. Biol. Chem. 286, 7535–7547 (2011).
Fischer, A.M., Katayama, C.D., Pages, G., Pouyssegur, J. & Hedrick, S.M. The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23, 431–443 (2005).
Daniels, M.A. et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 (2006).
Kane, L.P. & Hedrick, S.M. A role for calcium influx in setting the threshold for CD4+CD8+ thymocyte negative selection. J. Immunol. 156, 4594–4601 (1996).
Freedman, B.D., Liu, Q.H., Somersan, S., Kotlikoff, M.I. & Punt, J.A. Receptor avidity and costimulation specify the intracellular Ca2+ signaling pattern in CD4+CD8+ thymocytes. J. Exp. Med. 190, 943–952 (1999).
Lesourne, R. et al. Themis, a T cell-specific protein important for late thymocyte development. Nat. Immunol. 10, 840–847 (2009).
Fu, G. et al. Themis controls thymocyte selection through regulation of T cell antigen receptor–mediated signaling. Nat. Immunol. 10, 848–856 (2009).
Johnson, A.L. et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat. Immunol. 10, 831–839 (2009).
Cao, Y. et al. LKB1 regulates TCR-mediated PLCγ1 activation and thymocyte positive selection. EMBO J. 30, 2083–2093 (2011).
Boyden, L.M. et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 40, 656–662 (2008).
Diez-Roux, G. et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 9, e1000582 (2011).
Kurobe, H. et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity 24, 165–177 (2006).
Anderson, G. & Jenkinson, E.J. Investigating central tolerance with reaggregate thymus organ cultures. Methods Mol. Biol. 380, 185–196 (2007).
Brugnera, E. et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity 13, 59–71 (2000).
Lucas, B. & Germain, R.N. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity 5, 461–477 (1996).
Suzuki, H., Punt, J.A., Granger, L.G. & Singer, A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity 2, 413–425 (1995).
Cibotti, R., Punt, J.A., Dash, K.S., Sharrow, S.O. & Singer, A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity 6, 245–255 (1997).
Penninger, J.M. & Kroemer, G. Molecular and cellular mechanisms of T lymphocyte apoptosis. Adv. Immunol. 68, 51–144 (1998).
Bouillet, P. et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002).
Calnan, B.J., Szychowski, S., Chan, F.K., Cado, D. & Winoto, A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity 3, 273–282 (1995).
Yasutomo, K., Doyle, C., Miele, L., Fuchs, C. & Germain, R.N. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature 404, 506–510 (2000).
Itano, A. et al. The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J. Exp. Med. 183, 731–741 (1996).
Liu, X. & Bosselut, R. Duration of TCR signaling controls CD4–CD8 lineage differentiation in vivo. Nat. Immunol. 5, 280–288 (2004).
Amaral, M.C., Casillas, A.M. & Nel, A.E. Contrasting effects of two tumour promoters, phorbol myristate acetate and okadaic acid, on T-cell responses and activation of p42 MAP-kinase/ERK-2. Immunology 79, 24–31 (1993).
Hermiston, M.L. et al. Differential impact of the CD45 juxtamembrane wedge on central and peripheral T cell receptor responses. Proc. Natl. Acad. Sci. USA 106, 546–551 (2009).
Mayya, V. et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci. Signal. 2, ra46 (2009).
Zhang, W. et al. Essential role of LAT in T cell development. Immunity 10, 323–332 (1999).
Yachi, P.P., Ampudia, J., Gascoigne, N.R. & Zal, T. Nonstimulatory peptides contribute to antigen-induced CD8–T cell receptor interaction at the immunological synapse. Nat. Immunol. 6, 785–792 (2005).
Wang, D. et al. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc. Natl. Acad. Sci. USA 107, 13806–13811 (2010).
Acknowledgements
We thank X. Li, C. Xu and B. Li for discussions; H. Cantor, Y. Ke and H. Hu for critical reading; and A. Alison for assistance with manuscript editing. Supported by the National Natural Science Foundation of China (30972724 and 31070782 to L.L., and 30901311 and 31170842 to D.W.), the Zhejiang Provincial Natural Science Foundation of China (R2090202 to L.L., and Y2090401 to D.W.) and the National Basic Research Program of China (973 Program; 2011CB944100 and 2012CB945004 to L.L.).
Author information
Authors and Affiliations
Contributions
D.W., M.Z., L.W., Y.K., J.W., X.C. and L. Lu. designed research; D.W., M.Z., L. Lei., J.J., Y.Y., Y.Q., L.M., J.L., C.O., X.Z., Y.H., J.C. and L. Lu. did research; D.W., M.Z., L.W. and L. Lu. analyzed data; and D.W., M.Z. and L. Lu. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 4861 kb)
Supplementary Table 1
Genes differentially expressed in CD69+ Tespa1+/+ and Tespa1-/- thymocytes. (XLSX 291 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Zheng, M., Lei, L. et al. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat Immunol 13, 560–568 (2012). https://doi.org/10.1038/ni.2301
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2301
This article is cited by
-
Tespa1 facilitates hematopoietic and leukemic stem cell maintenance by restricting c-Myc degradation
Leukemia (2023)
-
Differential miRNA expression profiles in the bone marrow of Beagle dogs at different stages of Toxocara canis infection
BMC Genomics (2022)
-
Alcohol use disorder is associated with DNA methylation-based shortening of telomere length and regulated by TESPA1: implications for aging
Molecular Psychiatry (2022)
-
Mobilizing ER IP3 receptors as a mechanism to enhance calcium signaling
Cellular & Molecular Immunology (2021)
-
KRAP tethers IP3 receptors to actin and licenses them to evoke cytosolic Ca2+ signals
Nature Communications (2021)