Abstract
Chaperone-mediated autophagy (CMA) targets soluble proteins for lysosomal degradation. Here we found that CMA was activated in T cells in response to engagement of the T cell antigen receptor (TCR), which induced expression of the CMA-related lysosomal receptor LAMP-2A. In activated T cells, CMA targeted the ubiquitin ligase Itch and the calcineurin inhibitor RCAN1 for degradation to maintain activation-induced responses. Consequently, deletion of the gene encoding LAMP-2A in T cells caused deficient in vivo responses to immunization or infection with Listeria monocytogenes. Impaired CMA activity also occurred in T cells with age, which negatively affected their function. Restoration of LAMP-2A in T cells from old mice resulted in enhancement of activation-induced responses. Our findings define a role for CMA in regulating T cell activation through the targeted degradation of negative regulators of T cell activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mizushima, N. & Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 12, 823–830 (2010).
Cuervo, A.M. & Macian, F. Autophagy, nutrition and immunology. Mol. Aspects Med. 33, 2–13 (2012).
Dice, J.F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 15, 305–309 (1990).
Chiang, H.L., Terlecky, S.R., Plant, C.P. & Dice, J.F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246, 382–385 (1989).
Cuervo, A.M. & Dice, J.F. Unique properties of lamp2a compared to other lamp2 isoforms. J. Cell Sci. 113, 4441–4450 (2000).
Agarraberes, F.A., Terlecky, S.R. & Dice, J.F. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J. Cell Biol. 137, 825–834 (1997).
Cuervo, A.M. & Dice, J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273, 501–503 (1996).
Cuervo, A.M. & Dice, J.F. Regulation of lamp2a levels in the lysosomal membrane. Traffic 1, 570–583 (2000).
Kaushik, S., Massey, A.C. & Cuervo, A.M. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 25, 3921–3933 (2006).
Kiffin, R., Christian, C., Knecht, E. & Cuervo, A.M. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 15, 4829–4840 (2004).
Arias, E. & Cuervo, A.M. Chaperone-mediated autophagy in protein quality control. Curr. Opin. Cell Biol. 23, 184–189 (2011).
Zhang, C. & Cuervo, A.M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat. Med. 14, 959–965 (2008).
Pua, H.H., Dzhagalov, I., Chuck, M., Mizushima, N. & He, Y.W. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 204, 25–31 (2007).
Hubbard, V.M. et al. Macroautophagy regulates energy metabolism during effector T cell activation. J. Immunol. 185, 7349–7357 (2010).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Huan, C. et al. Transcription factors TFE3 and TFEB are critical for CD40 ligand expression and thymus-dependent humoral immunity. Nat. Immunol. 7, 1082–1091 (2006).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Koga, H. et al. Constitutive upregulation of chaperone-mediated autophagy in Huntington's disease. J. Neurosci. 31, 18492–18505 (2011).
Koga, H., Martinez-Vicente, M., Macian, F., Verkhusha, V.V. & Cuervo, A.M. A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat. Commun. 2, 386 (2011).
Cuervo, A.M., Dice, J.F. & Knecht, E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J. Biol. Chem. 272, 5606–5615 (1997).
Kwon, J. et al. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 3, ra59 (2010).
Sena, L.A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Macián, F. et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731 (2002).
Liu, H., Wang, P., Song, W. & Sun, X. Degradation of regulator of calcineurin 1 (RCAN1) is mediated by both chaperone-mediated autophagy and ubiquitin proteasome pathways. FASEB J. 23, 3383–3392 (2009).
Fuentes, J.J., Pritchard, M.A. & Estivill, X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics 44, 358–361 (1997).
Narayan, A.V. et al. Redox response of the endogenous calcineurin inhibitor Adapt 78. Free Radic. Biol. Med. 39, 719–727 (2005).
Schneider, J.L., Suh, Y. & Cuervo, A.M. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 20, 417–432 (2014).
Massey, A.C., Kaushik, S., Sovak, G., Kiffin, R. & Cuervo, A.M. Consequences of the selective blockage of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 103, 5805–5810 (2006).
Cuervo, A.M. & Dice, J.F. Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 275, 31505–31513 (2000).
Cuervo, A.M., Knecht, E., Terlecky, S.R. & Dice, J.F. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am. J. Physiol. 269, C1200–C1208 (1995).
Kon, M., Kiffin, R., Koga, H., Chapochnick, J. & Macian, F. et al. Chaperone-mediated autophagy is required for tumor growth. Sci. Transl. Med. 3, 109ra117 (2011).
Yang, Q. et al. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323, 124–127 (2009).
Pua, H.H., Guo, J., Komatsu, M. & He, Y.W. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 182, 4046–4055 (2009).
Jia, W., Pua, H.H., Li, Q.J. & He, Y.W. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J. Immunol. 186, 1564–1574 (2011).
Paul, S., Kashyap, A.K., Jia, W., He, Y.W. & Schaefer, B.C. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-κB. Immunity 36, 947–958 (2012).
Hubbard, V.M., Valdor, R., Macian, F. & Cuervo, A.M. Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology 13, 21–35 (2012).
Mueller, D.L. E3 ubiquitin ligases as T cell anergy factors. Nat. Immunol. 5, 883–890 (2004).
Fang, D. et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 3, 281–287 (2002).
Heissmeyer, V. et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5, 255–265 (2004).
Huang, H. et al. K33-linked polyubiquitination of T cell receptor-zeta regulates proteolysis-independent T cell signaling. Immunity 33, 60–70 (2010).
Yang, C. et al. Negative regulation of the E3 ubiquitin ligase itch via Fyn-mediated tyrosine phosphorylation. Mol. Cell 21, 135–141 (2006).
Zhang, J. et al. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J. Immunol. 169, 2236–2240 (2002).
Ryeom, S., Greenwald, R.J., Sharpe, A.H. & McKeon, F. The threshold pattern of calcineurin-dependent gene expression is altered by loss of the endogenous inhibitor calcipressin. Nat. Immunol. 4, 874–881 (2003).
Bhattacharyya, S. et al. NFATc1 affects mouse splenic B cell function by controlling the calcineurin–NFAT signaling network. J. Exp. Med. 208, 823–839 (2011).
Thompson, L.M. et al. IKK phosphorylates Huntingtin and targets it for degradation by the proteasome and lysosome. J. Cell Biol. 187, 1083–1099 (2009).
Cuervo, A.M., Hu, W., Lim, B. & Dice, J.F. IκB is a substrate for a selective pathway of lysosomal proteolysis. Mol. Biol. Cell 9, 1995–2010 (1998).
Chou, J.P. & Effros, R.B. T cell Replicative senescence in human aging. Curr. Pharm. Des. 19, 1680–1698 (2013).
Eisenbraun, M.D., Tamir, A. & Miller, R.A. Altered composition of the immunological synapse in an anergic, age-dependent memory T cell subset. J. Immunol. 164, 6105–6112 (2000).
Maue, A.C. et al. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 30, 301–305 (2009).
Goronzy, J.J. & Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436 (2013).
Barzilai, N. et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. J. Am. Med. Assoc. 290, 2030–2040 (2003).
Kim, S.H. et al. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br. J. Cancer 99, 741–749 (2008).
Chandra, D., Jahangir, A., Quispe-Tintaya, W., Einstein, M.H. & Gravekamp, C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br. J. Cancer 108, 2281–2290 (2013).
Storrie, B. & Madden, E.A. Isolation of subcellular organelles. Methods Enzymol. 182, 203–225 (1990).
Rubinsztein, D.C. et al. In search of an “autophagomometer”. Autophagy 5, 585–589 (2009).
Acknowledgements
We thank S. Ryeom (University of Pennsylvania) for the RCAN1 expression vector; G. Nolan (Stanford University) for Phoenix Ecotropic cells; S. Kaushik for help in the preparation of lysosomal fractions; and S. Bandyopadhyay for assistance in chromatin immunoprecipitation experiments. Supported by the US National Institutes of Health (AG031782), the Glenn Foundation (F.M. and A.M.C.) and the Einstein Nathan Shock Center in Basic Research in Aging (AG038072, for the use of specific research cores).
Author information
Authors and Affiliations
Contributions
R.V. designed and did experiments, analyzed data and wrote manuscript; E.M., Y.B., I.G.-R., D.C. and H.K. did experiments and contributed to data interpretation; C.G. designed experiments and interpreted data; A.M.C. designed project and interpreted data; and F.M. conceived of and directed project, interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
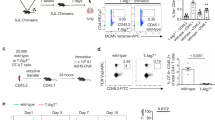
Supplementary Figure 1 Regulation of CMA during T cell activation.
(a). Resting (Rest) and activated (antiCD3+antiCD28) (Act) for 24 hours CD4+ T cells from and L2AcKO and control litter mate (WT) mice were stained with lysotracker to detect the lysosomal compartment. (b) Expression of TFEB was assessed by immunoblot in resting and activated (as in a) CD4+ T cells form WT and L2AcKO mice. Densitometric quantification of immunoblots from three different mice is also shown. (c-d) TH1 cells were transfected with a control non-targeting siRNA (Scr) or specific siRNAs for Tfeb (siT(1) and siT(2)). After 24 hours, TFEB expression was determined by immunoblot or real-time PCR (values, mean+s.e.m, are relative levels to untransfected control cells from three independent experiments). (e) TH1 cells were transfected with a control non-targeting siRNA (Scr) or a specific siRNA for Tfeb were activated for 24 hours as in a, and lysosomes visualized using lysotracker. A quantification (mean+s.e.m.) of cells from 2 different experiments (5 fields per experiment, 10-20 cells per field) is shown. (f-g) TH1 cells were transfected with non-targeting control siRNA (Scr) or siRNAs specific for Duox1 (siD(1) and siD(2)) (f) or Uqcrfs1 (siU(1)) (g). Twenty-four hours later expression of those genes was determined by real time PCR. Graphs show relative values to Scr-transfected cells (mean+s.e.m.) from two independent experiments performed in triplicate. (h) NFAT1 recruitment to the Lamp2 gene promoter was assessed by chromatin immunoprecipitation in resting naïve CD4+ T cells and in cells activated with PMA+ionomycin in the presence or absence of cyclosporine A (CsA). Graph shows mean+s.e.m. from two experiments.
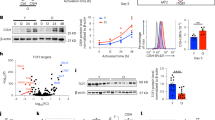
Supplementary Figure 2 Silencing Lamp2a mRNA expression impairs activation-induced proliferation and IL-2 expression in CD4+ T cells.
TH1 cells were transduced with lentiviral vectors expressing non-targeting control shRNA (shScr) or one of three different shRNAs specific for Lamp2a. Where indicated cells were also contransfected with a lentivirus expressing human LAMP-2A (hLamp2a). Forty-eight hours after transduction, cells were activated with antiCD3+antiCD28 for 24 hours and the expression of mouse Lamp2a mRNA (real-time PCR in (a)), IL-2 production (ELISA in (b)) and cell proliferation (BrdU incorporation in (c)) were measured. Graphs show mean+s.e.m. from two independent experiments performed in triplicate.
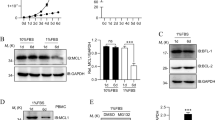
Supplementary Figure 3 Regulation of the degradation of Itch and Rcan1 by CMA.
(a) Cell lysates were prepared at different time points from TH1 cells activated with plate bound antiCD3 and antiCD28 (Act) and RCAN1 detected by immunoblot. β-actin was used as loading control. (b) TH1 cells were transfected with control empty plasmid (Ctrl) or plasmids expressing GFP-tagged versions of Itch or a mutant Itch with inactivated CMA-targeting motifs (mu-Itch), and cotransfected with a plasmid expressing GFP (used as an internal transfection efficiency control). Cells were activated for 24 hours as in a, and endogenous and plasmid-encoded tagged Itch were detected by immunoblot. (c) TH1 cells were transfected with control empty plasmid (Ctrl) or plasmids expressing Myc-tagged versions of RCAN1 or a mutant RCAN1 with inactivated CMA-targeting motifs (mu-RCAN1), and cotransfected with a plasmid expressing GFP (used as an internal transfection efficiency control). Cells were activated for 24 hours as in a, and endogenous and plasmid-encoded tagged RCAN1 were detected by immunoblot. (d-e) Experiments were performed as in b,c, and levels of wild type (WT) or KFERQ-mutant (KFERQ*) RCAN1 or Itch detected by immunoblot in resting (Rest) and activated T cells. (f) NIH3T3 cells were transfected with plasmids expressing Itch or a KFERQ-mutant form of Itch fused to GFP. After 24 h of serum starvation to activate CMA, localization of Itch in the lysosomal compartment was assessed marking lysosomes with 50 nM Lysotracker-red (Life Technologies) for the last 30 minutes of culture. Nuclei were stained with DAPI (blue). Original magnification 630x. (g) NIH3T3 cells were transfected with plasmids expressing wild type or a KFERQ-mutant form of Myc-tagged-RCAN1. After 24h of serum starvation to activate CMA, localization of RCAN1 in the lysosomal compartment was assessed using anti-Myc and anti-LAMP1 antibodies, Nuclei were stained with DAPI (blue). Original magnification 630x. (h) In vitro differentiated TH1 cell from L2AcKO mice and wild type littermates were co-transfected with plasmids expressing GFP and either wild type Myc-tagged RCAN1 or a CMA-targeting motifs mutated version of this protein. Cells were then activated for 24 hours and total cell lysates prepared to detect Myc-RCAN1, GFP and LAMP-2A by immunoblot. (i) Quantification (mean+SD) of mutated and wild type RCAN1 from three independent experiments as described in h.
Supplementary Figure 4 Characterization of the T cell compartment in LAMP-2A-deficient mice.
(a-c) Lysosome-enriched fractions were isolated from control litter mate mice (WT) or from LAMP-2A null mice (KO). LAMP-2A, LAMP-1 (a) and cathepsin D (b) were detected by immunoblot on lysosome-enriched fractions. Total β-hexosaminidase activity and lysosomal membrane stability, measured as the percentage of lysosomal β-hexosaminidase activity detectable in the media at the time of isolation (0 min) or upon incubation in an isotonic buffer for 20 min at 37°C, were also assessed. (c). Graphs show mean+s.e.m. of fractions obtained from three different mice per group. (d) Representative image of the thymus of L2AcKO mice and control littermates. Quantification of the total number of thymocytes per thymus is also shown. Results are mean+SD of 4 and 6 mice (t test, ** p<0.01). (e) Characterization by FACS of thymocyte populations from control littermate and L2AcKO mice (f) Distribution of CD4+, CD8+ and CD25+ populations in spleens of control littermate and L2AcKO mice was determined by FACS. Graph shows the average percentages (mean+s.e.m. from three mice) of CD4+ and CD8+ cells, as well as the percentage of CD25+ cells in the CD4+ population, in spleens from control littermate and L2AcKO mice. (g) Anti-OVA(323-339) immunoglobulin levels in serum of immunized control littermate and L2AcKO mice measured by ELISA using serial serum dilutions. Results are mean+s.e.m. from four different mice. (ANOVA with Tukey post test; *p<0.05; **p<0.01 between activated control littermate and L2AcKO T cells). h. Anti-Listeria immunoglobulin levels in serum of control littermate and L2AcKO mice previously infected with L. monocytogenes were measured by ELISA using serial serum dilutions. Results are mean+s.e.m. from three different mice.
Supplementary Figure 5 Signaling events in CD4+ T cells from LAMP-2A-deficient mice.
(a) Subcellular localization of NFAT1 was assessed by immunofluorescence in resting and plate bound anti-CD3 and anti-CD28-activated (20 min) CD4+ T cells isolated from control littermates (WT) or L2AcKO mice. (b-d) Cell lysates were prepared at different time points from CD4+ T cells isolated from control littermate or L2AcKO (KO) mice and then activated with plate bound anti-CD3 and anti-CD28. JNK phosphorylation (b), IκB degradation (c) and ERK phosphorylation (d) were measured by immunoblot. (e-f) Control littermate or L2AcKO mice CD4+ T cells were activated for 24 hours as in a. Localization of Itch (e) or (RCAN1) was assessed by immunofluorescence using specific antibodies for these proteins. Lysosomes were identified using anti-LAMP1 antibodies. Nuclei were stained with DAPI (blue). Original magnification 630x. (g) Naive and memory CD4+ T cells were isolated from 4 and 22 month old mice and activated for 24 hours a sin a. Itch and RCAN1 proteins were assessed by immunoblot, using tubulin as a loading control. Quantification of blots with samples obtained for four different animals for each age is shown. (h) CD4+ T cells isolated from 4 and 22 month old mice were activated for 12 hours as in a. ROS production was measured by FACS using CellROx-green.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 3384 kb)
Rights and permissions
About this article
Cite this article
Valdor, R., Mocholi, E., Botbol, Y. et al. Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat Immunol 15, 1046–1054 (2014). https://doi.org/10.1038/ni.3003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3003
This article is cited by
-
Exploiting autophagy balance in T and NK cells as a new strategy to implement adoptive cell therapies
Molecular Cancer (2023)
-
Chaperone-mediated autophagy in neurodegenerative diseases: mechanisms and therapy
Molecular and Cellular Biochemistry (2023)
-
Introducing Molecular Chaperones into the Causality and Prospective Management of Autoimmune Hepatitis
Digestive Diseases and Sciences (2023)
-
Loss of chaperone-mediated autophagy is associated with low vertebral cancellous bone mass
Scientific Reports (2022)
-
The immune synapses reveal aberrant functions of CD8 T cells during chronic HIV infection
Nature Communications (2022)