Abstract
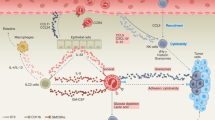
Tumor-associated eosinophilia is frequently observed in cancer. However, despite numerous studies of patients with cancer and mouse models of cancer, it has remained uncertain if eosinophils contribute to tumor immunity or are mere bystander cells. Here we report that activated eosinophils were essential for tumor rejection in the presence of tumor-specific CD8+ T cells. Tumor-homing eosinophils secreted chemoattractants that guided T cells into the tumor, which resulted in tumor eradication and survival. Activated eosinophils initiated substantial changes in the tumor microenvironment, including macrophage polarization and normalization of the tumor vasculature, which are known to promote tumor rejection. Thus, our study presents a new concept for eosinophils in cancer that may lead to novel therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
21 May 2015
In the version of this article initially published, the description of the data presented in Figure 4f–i was incorrect. That section of Results should read as follows: "The additional depletion of eosinophils...resulted in increased tumor hypoxia....(Fig. 4f and Supplementary Fig. 6). Depletion of eosinophils also increased vascular leakiness and diminished vascular perfusion...(Fig. 4g–i and Supplementary Fig. 6)." The errors have been corrected in the HTML and PDF versions of the article.
13 November 2015
In the version of this article initially published, the graph in Figure 2e was incorrect. This has been replaced with the correct graph. The error has been corrected in the HTML and PDF versions of the article.
References
Rothenberg, M.E. & Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 24, 147–174 (2006).
Rosenberg, H.F., Dyer, K.D. & Foster, P.S. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22 (2013).
Kita, H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol. Rev. 242, 161–177 (2011).
Melo, R.C., Liu, L., Xenakis, J.J. & Spencer, L.A. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy 68, 274–284 (2013).
Reinbach, G. Ueber das Verhalten der Leukocyten bei malignen Tumoren. Arch. Klin. Chir 46, 486–562 (1893).
Simson, L. et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J. Immunol. 178, 4222–4229 (2007).
Cormier, S.A. et al. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 79, 1131–1139 (2006).
Lotfi, R. et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 183, 5023–5031 (2009).
Lotfi, R., Lee, J.J. & Lotze, M.T. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J. Immunother. 30, 16–28 (2007).
Davis, B.P. & Rothenberg, M.E. Eosinophils and cancer. Cancer Immunol. Res. 2, 1–8 (2014).
Gatault, S., Legrand, F., Delbeke, M., Loiseau, S. & Capron, M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol. Immunother. 61, 1527–1534 (2012).
Tepper, R.I., Coffman, R.L. & Leder, P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257, 548–551 (1992).
Golumbek, P.T. et al. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science 254, 713–716 (1991).
Noffz, G., Qin, Z., Kopf, M. & Blankenstein, T. Neutrophils but not eosinophils are involved in growth suppression of IL-4-secreting tumors. J. Immunol. 160, 345–350 (1998).
Mattes, J. et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med. 197, 387–393 (2003).
Samoszuk, M. et al. Increased blood clotting, microvascular density, and inflammation in eotaxin-secreting tumors implanted into mice. Am. J. Pathol. 165, 449–456 (2004).
Krüger-Krasagakes, S., Li, W., Richter, G., Diamantstein, T. & Blankenstein, T. Eosinophils infiltrating interleukin-5 gene-transfected tumors do not suppress tumor growth. Eur. J. Immunol. 23, 992–995 (1993).
Huland, E. & Huland, H. Tumor-associated eosinophilia in interleukin-2-treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J. Cancer Res. Clin. Oncol. 118, 463–467 (1992).
Sosman, J.A. et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: effects of interleukin-4 alone and following interleukin-2 administration. Clin. Cancer Res. 1, 805–812 (1995).
Galdiero, M.R. et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 218, 1402–1410 (2013).
Li, X., Kostareli, E., Suffner, J., Garbi, N. & Hammerling, G.J. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur. J. Immunol. 40, 3325–3335 (2010).
Zimmermann, N. et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63, 1156–1163 (2008).
Chu, V.T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159 (2011).
Hamzah, J. et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453, 410–414 (2008).
Munitz, A. & Levi-Schaffer, F. Eosinophils: 'new' roles for 'old' cells. Allergy 59, 268–275 (2004).
Restifo, N.P., Dudley, M.E. & Rosenberg, S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Jain, R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Dalton, D.K. et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259, 1739–1742 (1993).
Stockmann, C. et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814–818 (2008).
Stockmann, C., Schadendorf, D., Klose, R. & Helfrich, I. The impact of the immune system on tumor: angiogenesis and vascular remodeling. Front. Oncol. 4, 69 (2014).
Huang, Y., Goel, S., Duda, D.G., Fukumura, D. & Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 73, 2943–2948 (2013).
Ganss, R., Ryschich, E., Klar, E., Arnold, B. & Hammerling, G.J. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 62, 1462–1470 (2002).
Ryschich, E., Schmidt, J., Hammerling, G.J., Klar, E. & Ganss, R. Transformation of the microvascular system during multistage tumorigenesis. Int. J. Cancer 97, 719–725 (2002).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475, 226–230 (2011).
Gabrilovich, D.I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013).
Garbi, N., Arnold, B., Gordon, S., Hammerling, G.J. & Ganss, R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J. Immunol. 172, 5861–5869 (2004).
Finlay, D.K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Shi, S. et al. Combining antiangiogenic therapy with adoptive cell immunotherapy exerts better antitumor effects in non-small cell lung cancer models. PLoS ONE 8, e65757 (2013).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 109, 17561–17566 (2012).
Liu, L.Y. et al. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-α. J. Immunol. 179, 4840–4848 (2007).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Delyon, J. et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 24, 1697–1703 (2013).
Motz, G.T. & Coukos, G. Deciphering and reversing tumor immune suppression. Immunity 39, 61–73 (2013).
Fridman, W.H. et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 71, 5601–5605 (2011).
Suffner, J. et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J. Immunol. 184, 1810–1820 (2010).
Kohlmeyer, J. et al. Complete regression of advanced primary and metastatic mouse melanomas following combination chemoimmunotherapy. Cancer Res. 69, 6265–6274 (2009).
Domschke, C. et al. Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Res. 69, 8420–8428 (2009).
Acknowledgements
We thank S. Schmitt for technical assistance; L. Umansky for multiplex analysis of cytokines; and T. Tüting (University of Bonn) for HCmel melanoma cells. Supported by Wilhelm Sander Stiftung (2009.022.2 to G.J.H.), the Cooperation Program in Cancer Research by the German Cancer Research Center, the Israel Ministry of Science, Technology and Space (G.J.H.) and the Bonn Cluster of Excellence to (N.G.).
Author information
Authors and Affiliations
Contributions
R.C., I.M.S., N.G., P.B. and G.J.H. designed the experimental plan; R.C., I.M.S. and O.C.S. performed the experiments; and R.C., I.M.S. and G.J.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
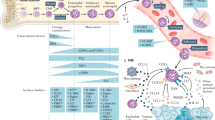
Supplementary Figure 1 Flow cytometry gating strategy for determination of tumor-infiltrating leukocyte subpopulations.
Abbreviations used: Singlets- single events. Leu- leukocytes. B- B cells. DCs- dendritic cells. Mye- Myeloid cells. NKT- NKT cells, NK- natural killer cells Neu- neutrophils. Mon- monocytes. Mac- macrophages. Eos- eosinophils. T- T cells. CD4- T helper cells. CD8- T cytotoxic cells.
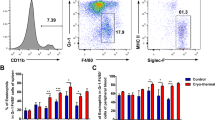
Supplementary Figure 2 Characterization of tumor-associated eosinophils.
a) Q-PCR analysis of sorted tumor CD11b+, Gr-1low, F4/80+ cells for Mbp and Epo eosinophil markers. b) Sorted tumor infiltrating eosinophils stained with hematoxilin-eosin after Treg depletion. Results are shown as mean ±SEM. n=6 mice per group. 1 of 3 independent experiments is shown. *=p<0.05; **=p<0.01; ***=p<0.001; NS= not significant (unpaired T-test).
Supplementary Figure 3 Efficiency of eosinophil depletion with mAb to Siglec-F.
1 day after intraperitoneal injection of Siglec-F mAb (15 ng/mouse) efficient depletion of eosinophils was observed in draining lymph nodes, (dLNs) and tumors. n=6 mice. 1 of 3 independent experiments is shown.
Supplementary Figure 4 Purification and survival of eosinophils.
a) Mice were injected i.p. with thioglycollate and peritoneal cells where harvested 3 days later. b) Eosinophils were purified from these peritoneal cells by MACS using beads coated with anti-PE antibodies and Siglec-F PE labeled antibodies. The purity obtained was above 80%. c) Enriched eosinophils were activated in vitro with complete RPMI medium containing IFNγ (15 ng/ml) and TNF (20 ng/ml) up to 16h. After activation the purity was 90%. d) Viability of eosinophil after in vitro culture. n=6. 1 of 3 independent experiments is shown.
Supplementary Figure 5 Titration of eosinophil-induced in vitro migration.
a) Multiplex protein analysis of lysates from activated or non-activated eosinophils. n=5. S Shown is 1 out of 2 independent experiments. b) Titration of eosinophil effect in CD8+ T cell in vitro migration. A range from 2,5x104 to 8x105 eosinophils in the lower chamber was used. Concentration of CD8+ T cells in the upper chamber was 1x105. c) Expression of angiogenesis factors by in vitro activated eosinophils. n=5 mice Results are shown as mean ±SEM. 1 of 2 independent experiments is shown. *=p<0.05; **=p<0.01; ***=p<0.001; NS= not significant (unpaired T-test).
Supplementary Figure 6 Examples of immunohistochemistry of tumors 6 d after depletion of Treg cells.
a) Hypoxia analysis. Hypoxiprobe (turquoise) and CD31 (magenta) staining. Bar =1mm b) Perfusion analysis. Tomatolectin (turquoise) and CD31 (magenta). Bar =100µm c) leakiness analysis. Dextran (turquoise) and CD31 (magenta). Bar =100µm d) Pericyte coverage. αSMA (turquoise) and CD31 (magenta). Bar =100µm. e-h) Quantification of IHC as shown in a-d. Each dot represents average of 10 fields evaluated per tumor. n=6 mice per group. Results are shown as mean ±SEM. *=p<0.05; **=p<0.01; ***=p<0.001; NS= not significant (unpaired T-test).
Supplementary Figure 7 Changes in the tumor microenvironment after transfer of eosinophils and OT-I cells.
a) Flow cytometric quantification of tumor infiltrating leukocyte subpopulations after transfer of activated eosinophils and OTI cells. The data shows significantly more T-cells infiltrating the tumor after cotransfer of activated eosinophils and OT-I as compamagenta to OTI cells alone. b) Representative confocal images of MO4 tumors 2 days after transfer of activated tumor-specific CD8+ T cells and activated eosinophils. Bar, 50µm. n=7 mice per group. c) Q-PCR analysis of cytokine and chemokine levels in the tumor microenvironment after transfer of activated eosinophils and OTI cells. d) Q-PCR analysis of angiogenesis factors in the tumor microenvironment after transfer of activated eosinophils and OTI cells. n=6 mice. Results are shown as mean ±SEM. 1 of 2 independent experiments is shown *=p<0.05; **=p<0.01; ***=p<0.001; NS=not significant (unpaired T-test).
Supplementary Figure 8 Effect of eosinophils in the Hcmel melanoma model.
a) Tumor growth after adoptive therapy of 5x106 eosinophils and 5x106 pmel CD8+ T cells. b) Survival of mice shown in a. n=6 mice. Results are shown as mean ±SEM. 1 of 2 independent experiments is shown. *=p<0.05; **=p<0.01; ***=p<0.001; ns= not significant. log Rank (Mantel-Cox) test.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 2247 kb)
Rights and permissions
About this article
Cite this article
Carretero, R., Sektioglu, I., Garbi, N. et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat Immunol 16, 609–617 (2015). https://doi.org/10.1038/ni.3159
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3159
This article is cited by
-
How chemokines organize the tumour microenvironment
Nature Reviews Cancer (2024)
-
Neutrophil-to-lymphocyte ratio and monocyte-to-eosinophil ratio as prognostic indicators for advanced nasopharyngeal carcinoma
European Archives of Oto-Rhino-Laryngology (2024)
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)
-
Eosinophils in the tumor microenvironment: implications for cancer immunotherapy
Journal of Translational Medicine (2023)
-
Alleviating hypoxia to improve cancer immunotherapy
Oncogene (2023)