Abstract
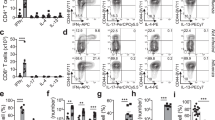
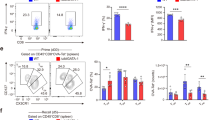
Type 2 helper T cells (TH2 cells) produce interleukin 13 (IL-13) when stimulated by papain or house dust mite extract (HDM) and induce eosinophilic inflammation. This innate response is dependent on IL-33 but not T cell antigen receptors (TCRs). While type 2 innate lymphoid cells (ILC2 cells) are the dominant innate producers of IL-13 in naive mice, we found here that helminth-infected mice had more TH2 cells compared to uninfected mice, and thes e cells became major mediators of innate type 2 responses. TH2 cells made important contributions to HDM-induced antigen-nonspecific eosinophilic inflammation and protected mice recovering from infection with Ascaris suum against subsequent infection with the phylogenetically distant nematode Nippostrongylus brasiliensis. Our findings reveal a previously unappreciated role for effector TH2 cells during TCR-independent innate-like immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Janeway, C.A. Jr. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Spits, H. & Cupedo, T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30, 647–675 (2012).
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Guo, L. et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA 106, 13463–13468 (2009).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1 cells are multipotential 'inflammatory' type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Wang, Z. et al. Systematic analysis of insertions and deletions specific to nematode proteins and their proposed functional and evolutionary relevance. BMC Evol. Biol. 9, 23–36 (2009).
Halim, T.Y., Krauss, R.H., Sun, A.C. & Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36, 451–463 (2012).
Wong, S.H. et al. Transcription factor RORα is critical for nuocyte development. Nat. Immunol. 13, 229–236 (2012).
Pichery, M. et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 188, 3488–3495 (2012).
Saenz, S.A., Taylor, B.C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008).
Cayrol, C. & Girard, J.P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 31, 31–37 (2014).
Kakkar, R. & Lee, R.T. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 7, 827–840 (2008).
Liew, F.Y., Pitman, N.I. & McInnes, I.B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 10, 103–110 (2010).
Ziegler, S.F. & Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 11, 289–293 (2010).
McKenzie, G.J., Bancroft, A., Grencis, R.K. & McKenzie, A.N. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8, 339–342 (1998).
Ramalingam, T.R. et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat. Immunol. 9, 25–33 (2008).
Grünig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261–2263 (1998).
Wills-Karp, M. et al. Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 (1998).
Guo, L., Junttila, I.S. & Paul, W.E. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 33, 598–606 (2012).
Erb, K.J. Can helminths or helminth-derived products be used in humans to prevent or treat allergic diseases? Trends Immunol. 30, 75–82 (2009).
van Riet, E., Hartgers, F.C. & Yazdanbakhsh, M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212, 475–490 (2007).
Palmer, L.J. et al. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am. J. Resp. Crit. Care Med. 165, 1489–1493 (2002).
Buijs, J. et al. Relationship between allergic manifestations and Toxocara seropositivity: a cross-sectional study among elementary school children. Eur. Respir. J. 10, 1467–1475 (1997).
Pinelli, E., Brandes, S., Dormans, J., Gremmer, E. & van Loveren, H. Infection with the roundworm Toxocara canis leads to exacerbation of experimental allergic airway inflammation. Clin. Exp. Allergy 38, 649–658 (2008).
Lynch, N.R., Palenque, M., Hagel, I. & DiPrisco, M.C. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am. J. Resp. Crit. Care Med. 156, 50–54 (1997).
Yazdanbakhsh, M., van den Biggelaar, A. & Maizels, R.M. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 22, 372–377 (2001).
Guarner, F. et al. Mechanisms of disease: the hygiene hypothesis revisited. Nat. Clin. Pract .Gastroenterol. Hepatol. 3, 275–284 (2006).
Schopf, L. et al. Differential modulation of allergic eye disease by chronic and acute ascaris infection. Invest. Ophthalmol. Vis. Sci. 46, 2772–2780 (2005).
Harnett, W. & Harnett, M.M. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat. Rev. Immunol. 10, 278–284 (2010).
Anthony, R.M., Rutitzky, L.I., Urban, J.F. Jr., Stadecker, M.J. & Gause, W.C. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7, 975–987 (2007).
Fallon, P.G. & Mangan, N.E. Suppression of TH2-type allergic reactions by helminth infection. Nat. Rev. Immunol. 7, 220–230 (2007).
Yazdanbakhsh, M., Kremsner, P.G. & van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494 (2002).
Shin, E.H. et al. Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int. Arch. Allergy Immunol. 114, 45–50 (1997).
Knott, M.L. et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int. J. Parasitol. 37, 1367–1378 (2007).
Schwartz, C. et al. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc. Natl. Acad. Sci. USA 111, E5169–E5177 (2014).
Chen, F. et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946 (2014).
Townsend, M.J., Fallon, P.G., Matthews, D.J., Jolin, H.E. & McKenzie, A.N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 (2000).
Acknowledgements
We thank A.N. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK) for Il1rl1−/− mice; J.-P. Girard (Centre National de la Recherche Scientifique, Institute de Pharmacology et de Biologie Structurale) for Il33-LacZ gene-trap reporter mice; J. Ward for help with pathology analysis; and J. Edwards and K. Weng for help with cell sorting. Supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases (US National Institute of Health).
Author information
Authors and Affiliations
Contributions
L.G. designed, performed and interpreted experiments and wrote the manuscript; Y.H., X.C. and J.H.-L. assisted with some experiments; J.F.U. helped to design, perform and interpret experiments involving infection with A. suum and N. brasiliensis, provided N. brasiliensis, and read the manuscript; and W.E.P. designed and interpreted the experiments, wrote the manuscript and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The expression of fluorescent proteins in 4C13R dual-reporter mice is a reliable reporter of cytokine production.
Naïve CD4 T cells were sorted from 4C13R dual reporter mice and cultured under TH2 conditions (a,b) or TH17 conditions (c) for two rounds. Cells were stimulated with PMA plus ionomycin or IL-33 plus IL-7 for 4 hours in the presence of monensin. Cytokine production was measured by intracellular staining. Data are representative of at least three independent experiments.
Supplementary Figure 2 Administration of IL-33 and TSLP induces TH2 cells to produce IL-13.
(a) Naïve CD4 T cells were sorted from OT-II–4C13R reporter mice and cultured under TH2 conditions for three rounds. Cells were rested in IL-7-containing medium for 10 days and then injected i.v. into wild-type C57BL/6 recipient mice. Twenty-four hours later, mice were intratracheally challenged with PBS or indicated cytokines (150 ng IL-33 and/or 150 ng TSLP each mouse) for 3 consecutive days, or OVA (100 μg endotoxin-free OVA in PBS) once. Lungs were collected and cytokine production was examined 24h after last cytokine administration. Cells shown were gated on transferred OT-II TH2 cells. (b) Statistical analysis of the cytokine production. Error bars represent standard deviation from the mean. ****, P<0.0001; ns, P>0.05 by two tailed student’s t-test. Data are representative of two independent experiments with 4 mice in each group.
Supplementary Figure 3 Antigen-induced expression of DsRed is blocked by anti–MHC class II in FcRγ-deficient mice.
(a, b) 0.5x106 naïve CD4 cells from OT-II–4C13R reporter mice were transferred i.v. into FcRγ chain-deficient mice. Mice were immunized and (hallenged with OVA once as in Fig. 2a. In some mice, 500 μg anti-MHCII antibody was administered i.v. on Day 1 and Day 3 of OVA stimulation. Lungs were collected 72h after OVA challenge; DsRed and AmCyan expression by transferred OT-II TH2 cells were analyzed. (b) Statistical analysis of the cytokine production by the transferred OT-II TH2 cells. *, P<0.05; **, P<0.01. Data are representative of one experiment with 3 mice in each group.
Supplementary Figure 4 Wild-type TH2 cells and Il1rl1−/− TH2 cells produce similar amounts of cytokines in response to stimulation with PMA plus ionomycin.
Naïve CD4 T cells were sorted from wild-type (WT) CD90.1+ CD90.2+ DO11.10 mice or Il1rl1−/− CD90.1–CD90.2+ DO11.10 mice and cultured under TH2 conditions for three rounds. (a) In vitro cultured WT TH2 cells and Il1rl1−/− TH2 cells were stimulated with PMA plus ionomycin for 4h. Cytokine production was examined by intracellular staining. (b) IL-33R expression on the WT and Il1rl1−/− TH2 cells was examined. Data are representative of two experiments with 2-3 mice in each group.
Supplementary Figure 5 Previous infection with A. suum. protects mice against a subsequent infection with N. brasiliensis.
(a) C57BL/6 mice were inoculated with A. summ eggs orally. After the indicated periods, mice received a subsequent subcutaneous inoculation with 500 N. brasiliensis L3. The mice in control group were only inoculated subcutaneously with 500 N. brasiliensis L3. (b) N. brasiliensis adult worms in the small intestine were determined on day 8 post N. brasiliensis inoculation. ***, P<0.001. Data are representative of one experiment with three mice in each group.
Supplementary Figure 6 Substantial reduction in ILC2 cells in chimeras reconstituted with RORα-deficient BM.
8 weeks after bone marrow reconstitution, lungs from WT or RORα-deficient BM chimeras were collected and single cell suspension was prepared. Frequency of lung-resident ILC2 (live Lin–CD127+GATA3+RORγt– lymphocytes) and ILC3 (live Lin–CD127+GATA3–RORγt+ lymphocytes) cells were analyzed in two individual mice from each group. Data are representative of one experiment with two mice in each group.
Supplementary Figure 7 Induction of ILC2 cells in BM chimeras.
WT or RORα-deficient BM chimeras were infected as Fig. 7g. In some mice (c), mice were i.v. injected with 500 μg anti-CD4 antibody on Day1 and Day4 of N. brasiliensis inoculation. (a) Proportion of lung-resident ILC2 cells in WT and RORα-deficient BM chimeras 8 days after they received N. brasiliensis inoculation. (b-d) WT and RORα-deficient BM chimeras that have been inoculated with A. suum and subsequently with N.brasiliensis were analyzed 8 days post N. brasiliensis inoculation. Adult N. brasiliensis worm burden in intestine vs the proportion of the lung-resident ILC2 cells were plotted. Data are compiled from three independent experiments with 4-5 mice each group.
Supplementary Figure 8 Wild-type and RORα-deficient TH2 cells produce similar amounts of cytokines in response to PMA plus ionomycin or IL-33 plus IL-7.
Naïve CD4 T cells were sorted from WT or RORα-deficient BM chimeras. T cells were cultured under TH2 conditions with anti-CD3 and anti-CD28 in the presence of APC for two rounds. To test cytokine production, cells were stimulated with (a) PMA and ionomycin or (b) IL-33+IL-7 in the presence of monensin for 4h. Cytokine production was examined by intracellular staining. Data are representative of two independent experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 1191 kb)
Rights and permissions
About this article
Cite this article
Guo, L., Huang, Y., Chen, X. et al. Innate immunological function of TH2 cells in vivo. Nat Immunol 16, 1051–1059 (2015). https://doi.org/10.1038/ni.3244
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3244
This article is cited by
-
Cooperation of ILC2s and TH2 cells in the expulsion of intestinal helminth parasites
Nature Reviews Immunology (2024)
-
Biological function of resveratrol and its application in animal production: a review
Journal of Animal Science and Biotechnology (2023)
-
Steady-state memory-phenotype conventional CD4+ T cells exacerbate autoimmune neuroinflammation in a bystander manner via the Bhlhe40/GM-CSF axis
Experimental & Molecular Medicine (2023)
-
Dynamic chromatin accessibility licenses STAT5- and STAT6-dependent innate-like function of TH9 cells to promote allergic inflammation
Nature Immunology (2023)
-
Significance of bystander T cell activation in microbial infection
Nature Immunology (2022)