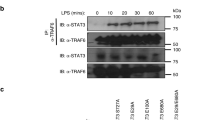
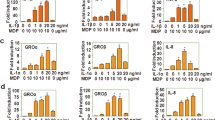
Abstract
The cellular mechanisms that directly regulate the inflammatory response after Toll-like receptor (TLR) stimulation are unresolved at present. Here we report that glycogen synthase kinase 3 (GSK3) differentially regulates TLR-mediated production of pro- and anti-inflammatory cytokines. Stimulation of monocytes or peripheral blood mononuclear cells with TLR2, TLR4, TLR5 or TLR9 agonists induced substantial increases in interleukin 10 production while suppressing the release of proinflammatory cytokines after GSK3 inhibition. GSK3 regulated the inflammatory response by differentially affecting the nuclear amounts of transcription factors NF-κB subunit p65 and CREB interacting with the coactivator CBP. Administration of a GSK3 inhibitor potently suppressed the proinflammatory response in mice receiving lipopolysaccharide and mediated protection from endotoxin shock. These findings demonstrate a regulatory function for GSK3 in modulating the inflammatory response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Yang, R.B. et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395, 284–288 (1998).
Dinarello, C.A. Proinflammatory cytokines. Chest 118, 503–508 (2000).
O'Neill, L.A. & Dinarello, C.A. The IL-1 receptor/Toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol. Today 21, 206–209 (2000).
Monick, M.M. et al. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J. Immunol. 166, 4713–4720 (2001).
Fukao, T. et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3, 875–881 (2002).
Fukao, T. et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat. Immunol. 3, 295–304 (2002).
Guha, M. & Mackman, N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277, 32124–32132 (2002).
Martin, M. et al. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 171, 717–725 (2003).
Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Arbibe, L. et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 1, 533–540 (2000).
Toker, A. & Cantley, L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387, 673–676 (1997).
Franke, T.F., Kaplan, D.R., Cantley, L.C. & Toker, A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275, 665–668 (1997).
Lawlor, M.A. & Alessi, D.R. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114, 2903–2910 (2001).
Stokoe, D.L.R. et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 (1997).
Cross, D.A., Alessi, D.R., Cohen, P., Andjelkovich, M. & Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Hoeflich, K.P. et al. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406, 86–90 (2000).
Demarchi, F., Bertoli, C., Sandy, P. & Schneider, C. Glycogen synthase kinase-3 β regulates NF-κB1/p105 stability. J. Biol. Chem. 278, 39583–39590 (2003).
Demarchi, F., Verardo, R., Varnum, B., Brancolini, C. & Schneider, C. Gas6 anti-apoptotic signaling requires NF-κB activation. J. Biol. Chem. 276, 31738–31744 (2001).
Nemeth, Z.H. et al. Lithium induces NF-κB activation and interleukin-8 production in human intestinal epithelial cells. J. Biol. Chem. 277, 7713–7719 (2002).
Schwabe, R.F. & Brenner, D.A. Role of glycogen synthase kinase-3 in TNF-α-induced NF-κB activation and apoptosis in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G204–G211 (2002).
Grimes, C.A. & Jope, R.S. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 β and facilitated by lithium. J. Neurochem. 78, 1219–1232 (2001).
Doble, B.W. & Woodgett, J.R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003).
Kunick, C., Lauenroth, K., Leost, M., Meijer, L. & Lemcke, T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3 β. Bioorg. Med. Chem. Lett. 19, 413–416 (2004).
Cross, D.A. et al. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 77, 94–102 (2001).
Meijer, L. et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255–1266 (2003).
Stambolic, V., Ruel, L. & Woodgett, J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668 (1996).
Woodgett, J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 9, 2431–2438 (1990).
Ghosh, S., May, M.J. & Kopp, E.B. NF-κB and rel proteins: evolutionary conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
Sheppard, K.A. et al. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19, 6367–6378 (1999).
Zhong, H., Voll, R.E. & Ghosh, S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1, 661–671 (1998).
Parker, D. et al. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol. Cell. Biol. 16, 694–703 (1996).
Parry, G.C. & Mackman, N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J. Immunol. 159, 5450–5456 (1997).
Platzer, C. et al. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 29, 3098–3104 (1999).
Berg, D.J. et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Invest. 96, 2339–2347 (1995).
Hirschfeld, M. et al. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69, 1477–1482 (2001).
Jones, B.W. et al. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leuk. Biol. 69, 1036–1044 (2001).
Re, F. & Strominger, J.L. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276, 37692–37699 (2001).
Rhee, S.H., Jones, B.W., Toshchakov, V., Vogel, S.N. & Fenton, M.J. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J. Biol. Chem. 278, 22506–22512 (2003).
Schilling, D., Thomas, K., Nixdorff, K., Vogel, S.N. & Fenton, M.J. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J. Immunol. 169, 5874–5880 (2002).
Toshchakov, V. et al. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3, 392–398 (2002).
Dillon, S. et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172, 4733–4743 (2004).
Re, F. & Strominger, J.L. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J. Immunol. 173, 7548–7555 (2004).
Pulendran, B. et al. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167, 5067–5076 (2001).
Cohen, J. The immunopathogenesis of sepsis. Nature 420, 885–891 (2002).
O'Brien, W.T. et al. Glycogen synthase kinase-3β haploinsufficiency mimics the behavioral and molecular effects of lithium. J. Neurosci. 24, 6791–6798 (2004).
Hirschfeld, M., Ma, Y., Weis, J.H., Vogel, S.N. & Weis, J.J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165, 18–22 (2000).
Tapping, R.I., Akashi, S., Miyake, K., Godowski, P.J. & Tobias, P.S. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165, 5780–5787 (2000).
Acknowledgements
Supported in part by the US Public Health Service (DE 08182, DE 14215, DE 09081 and AI 056460).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Martin, M., Rehani, K., Jope, R. et al. Toll-like receptor–mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 6, 777–784 (2005). https://doi.org/10.1038/ni1221
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1221
This article is cited by
-
α7 Nicotinic acetylcholine receptor: a key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect
Journal of Neuroinflammation (2023)
-
Low-dose lithium adjunct to atypical antipsychotic treatment nearly improved cognitive impairment, deteriorated the gray-matter volume, and decreased the interleukin-6 level in drug-naive patients with first schizophrenia symptoms: a follow-up pilot study
Schizophrenia (2023)
-
Understanding Glycogen Synthase Kinase-3: A Novel Avenue for Alzheimer’s Disease
Molecular Neurobiology (2023)
-
Sepsis-associated brain injury: underlying mechanisms and potential therapeutic strategies for acute and long-term cognitive impairments
Journal of Neuroinflammation (2022)
-
Drug delivery of 6-bromoindirubin-3’-glycerol-oxime ether employing poly(d,l-lactide-co-glycolide)-based nanoencapsulation techniques with sustainable solvents
Journal of Nanobiotechnology (2022)