Abstract
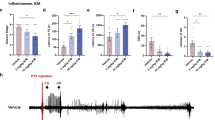
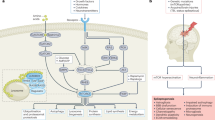
Brain inflammation is a major factor in epilepsy, but the impact of specific inflammatory mediators on neuronal excitability is incompletely understood. Using models of acute and chronic seizures in C57BL/6 mice, we discovered a proconvulsant pathway involving high-mobility group box-1 (HMGB1) release from neurons and glia and its interaction with Toll-like receptor 4 (TLR4), a key receptor of innate immunity. Antagonists of HMGB1 and TLR4 retard seizure precipitation and decrease acute and chronic seizure recurrence. TLR4-defective C3H/HeJ mice are resistant to kainate-induced seizures. The proconvulsant effects of HMGB1, like those of interleukin-1β (IL-1β), are partly mediated by ifenprodil-sensitive N-methyl-d-aspartate (NMDA) receptors. Increased expression of HMGB1 and TLR4 in human epileptogenic tissue, like that observed in the mouse model of chronic seizures, suggests a role for the HMGB1-TLR4 axis in human epilepsy. Thus, HMGB1-TLR4 signaling may contribute to generating and perpetuating seizures in humans and might be targeted to attain anticonvulsant effects in epilepsies that are currently resistant to drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hauser, W.A., Annegers, J.F. & Kurland, L.T. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 34, 453–468 (1993).
Perucca, E., French, J. & Bialer, M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 6, 793–804 (2007).
Vezzani, A. & Granata, T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46, 1724–1743 (2005).
Pitkänen, A. & Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 1, 173–181 (2002).
Bartfai, T. et al. Interleukin-1 system in CNS stress: seizures, fever and neurotrauma. Ann. NY Acad. Sci. 1113, 173–177 (2007).
Sheng, J.G., Boop, F.A., Mrak, R.E. & Griffin, W.S. Increased neuronal β-amyloid precursor protein expression in human temporal lobe epilepsy: association with interleukin-1α immunoreactivity. J. Neurochem. 63, 1872–1879 (1994).
Crespel, A. et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 952, 159–169 (2002).
Ravizza, T. et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 29, 142–160 (2008).
Ravizza, T. et al. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol. Dis. 24, 128–143 (2006).
Boer, K. et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 78, 7–21 (2008).
Vezzani, A., Balosso, S. & Ravizza, T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav. Immun. 22, 797–803 (2008).
Vezzani, A. et al. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J. Neurosci. 19, 5054–5065 (1999).
De Simoni, M.G. et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur. J. Neurosci. 12, 2623–2633 (2000).
Ravizza, T. et al. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol. Dis. 31, 327–333 (2008).
Viviani, B. et al. Interleukin-1β enhances NMDA receptor–mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 23, 8692–8700 (2003).
Balosso, S. et al. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1β. Brain 131, 3256–3265 (2008).
Kawai, T. & Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 13, 460–469 (2007).
Chakravarty, S. & Herkenham, M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 25, 1788–1796 (2005).
Sayyah, M., Javad-Pour, M. & Ghazi-Khansari, M. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice: involvement of proinflammatory factors: nitric oxide and prostaglandins. Neuroscience 122, 1073–1080 (2003).
Galic, M.A. et al. Postnatal inflammation increases seizure susceptibility in adult rats. J. Neurosci. 28, 6904–6913 (2008).
Bianchi, M.E. & Manfredi, A.A. Immunology. Dangers in and out. Science 323, 1683–1684 (2009).
Scaffidi, P., Misteli, T. & Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Müller, S., Ronfani, L. & Bianchi, M.E. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J. Intern. Med. 255, 332–343 (2004).
Bonaldi, T. et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22, 5551–5560 (2003).
Park, J.S. et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279, 7370–7377 (2004).
Apetoh, L. et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Ravizza, T. et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia 47, 1160–1168 (2006).
Vezzani, A. et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl. Acad. Sci. USA 97, 11534–11539 (2000).
Riban, V. et al. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience 112, 101–111 (2002).
Bouilleret, V. et al. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 89, 717–729 (1999).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Trisciuoglio, L. & Bianchi, M.E. Several nuclear events during apoptosis depend on caspase-3 activation but do not constitute a common pathway. PLoS One 4, e6234 (2009).
Yu, S.W. et al. Mediation of poly(ADP-ribose) polymerase-1–dependent cell death by apoptosis-inducing factor. Science 297, 259–263 (2002).
Yang, H. et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 101, 296–301 (2004).
Sitia, G., Iannacone, M., Muller, S., Bianchi, M.E. & Guidotti, L.G. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J. Leukoc. Biol. 81, 100–107 (2007).
Muhammad, S. et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J. Neurosci. 28, 12023–12031 (2008).
Kutuzova, G.D., Albrecht, R.M., Erickson, C.M. & Qureshi, N. Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW 264.7 cells. J. Immunol. 167, 482–489 (2001).
Macagno, A. et al. A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression. J. Exp. Med. 203, 1481–1492 (2006).
Chenard, B.L. & Menniti, F.S. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr. Pharm. Des. 5, 381–404 (1999).
Ebert, U., Wlaz, P. & Loscher, W. Anticonvulsant effects by combined treatment with a glycineB receptor antagonist and a polyamine site antagonist in amygdala-kindled rats. Eur. J. Pharmacol. 322, 179–184 (1997).
Yourick, D.L., Repasi, R.T., Rittase, W.B., Staten, L.D. & Meyerhoff, J.L. Ifenprodil and arcaine alter amygdala-kindling development. Eur. J. Pharmacol. 371, 147–152 (1999).
De Sarro, G.B., Bagetta, G., Spagnolo, C. & Nistico, G. Antagonists of N-methyl-d-aspartate receptors block seizures induced by putrescine in the deep prepiriform cortex. Neuropharmacology 32, 43–50 (1993).
Lee, M.S. & Kim, Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76, 447–480 (2007).
Rodgers, K.M. et al. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain 132, 2478–2486 (2009).
Singh, G., Prabhakar, S. & Modi, M. Central nervous system infections and epilepsy. Epilepsia 49 Suppl 6, 1 (2008).
Prince, D.A. et al. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia 50 Suppl 2, 30–40 (2009).
Yu, X.M., Askalan, R., Keil, G.J. II & Salter, M.W. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275, 674–678 (1997).
Tsung, A. et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204, 2913–2923 (2007).
Andrassy, M. et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117, 3216–3226 (2008).
Zhao, M., Ma, H., Suh, M. & Schwartz, T.H. Spatiotemporal dynamics of perfusion and oximetry during ictal discharges in the rat neocortex. J. Neurosci. 29, 2814–2823 (2009).
Ishida, I. et al. Hypoxia diminishes Toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J. Immunol. 169, 2069–2075 (2002).
Yang, H., Wang, H., Czura, C.J. & Tracey, K.J. HMGB1 as a cytokine and therapeutic target. J. Endotoxin Res. 8, 469–472 (2002).
Kralic, J.E., Ledergerber, D.A. & Fritschy, J.M. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur. J. Neurosci. 22, 1916–1927 (2005).
Suzuki, F. et al. Glutamate receptor antagonists and benzodiazepine inhibit the progression of granule cell dispersion in a mouse model of mesial temporal lobe epilepsy. Epilepsia 46, 193–202 (2005).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates. (Academic Press, San Diego, 1997).
Acknowledgements
This work was supported by a CARIPLO Foundation grant (A.V., M.E.B. and C.R.), by Parents Against Childhood Epilepsy (A.V.), Associazione Italiana Contro l'Epilessia (T.R.), EU FP6 project EPICURE (LSH-CT-2006-037315) (A.V.), EU FP7 project NeuroGlia (No. 202167) and National Epilepsy Fund (NEF 09-05) (E.A.) and a grant from Regione Lombardia to HMGBiotech. J.L. is a recipient of an EU fellowship in the International Graduate Programme in Molecular Medicine training program. We acknowledge F. De Ceglie for technical support in photograph preparation.
Author information
Authors and Affiliations
Contributions
M. Maroso and S.B. conducted the pharmacological studies in the experimental models of seizures and performed the analysis of data; T.R. conducted the immunohistochemical analysis in the experimental models and analyzed the data; J.L. performed the western blot analyses and in vitro experiments; E.A. and A.M.I. conducted the immunohistochemical studies in human tissues and data analysis; C.R. and M. Molteni characterized and provided the TLR4 antagonists used in the pharmacological experiments; M.C. produced HMGB1 and BoxA; A.A.M. contributed experimental suggestions and participated in manuscript editing; M.E.B. together with A.V. supervised all phases of the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Declaration: M.M. is employed by and has personal financial interests in Bluegreen Biotech. M.E.B. is founder and part-owner of HMGBiotech, a biotech that sells products and idea based on the functions of HMGB proteins. M.C. is an employee of HMGBiotech.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–7 (PDF 889 kb)
Rights and permissions
About this article
Cite this article
Maroso, M., Balosso, S., Ravizza, T. et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16, 413–419 (2010). https://doi.org/10.1038/nm.2127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2127
This article is cited by
-
Increased expression of NLRP3 associated with elevated levels of HMGB1 in children with febrile seizures: a case–control study
BMC Pediatrics (2024)
-
Inflammasomes in neurological disorders — mechanisms and therapeutic potential
Nature Reviews Neurology (2024)
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment
Nature Reviews Neuroscience (2024)
-
Toll-Like Receptor 4 Deficiency Ameliorates Propofol-Induced Impairments of Cognitive Function and Synaptic Plasticity in Young Mice
Molecular Neurobiology (2024)
-
The Emerging Role of Toll-Like Receptor-Mediated Neuroinflammatory Signals in Psychiatric Disorders and Acquired Epilepsy
Molecular Neurobiology (2024)