Abstract
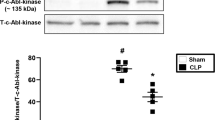
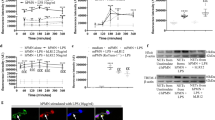
Sepsis is a systemic inflammatory condition following bacterial infection with a high mortality rate and limited therapeutic options1,2. Here we show that interleukin-33 (IL-33) reduces mortality in mice with experimental sepsis from cecal ligation and puncture (CLP). IL-33–treated mice developed increased neutrophil influx into the peritoneal cavity and more efficient bacterial clearance than untreated mice. IL-33 reduced the systemic but not the local proinflammatory response, and it did not induce a T helper type 1 (TH1) to TH2 shift. The chemokine receptor CXCR2 is crucial for recruitment of neutrophils from the circulation to the site of infection3. Activation of Toll-like receptors (TLRs) in neutrophils downregulates CXCR2 expression and impairs neutrophil migration4. We show here that IL-33 prevents the downregulation of CXCR2 and inhibition of chemotaxis induced by the activation of TLR4 in mouse and human neutrophils. Furthermore, we show that IL-33 reverses the TLR4-induced reduction of CXCR2 expression in neutrophils via the inhibition of expression of G protein–coupled receptor kinase-2 (GRK2), a serine-threonine protein kinase that induces internalization of chemokine receptors5,6. Finally, we find that individuals who did not recover from sepsis had significantly more soluble ST2 (sST2, the decoy receptor of IL-33) than those who did recover. Together, our results indicate a previously undescribed mechanism of action of IL-33 and suggest a therapeutic potential of IL-33 in sepsis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hotchkiss, R.S. & Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 (2003).
Riedemann, N.C., Guo, R.F. & Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 9, 517–524 (2003).
Olson, T.S. & Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R7–R28 (2002).
Alves-Filho, J.C. et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. USA 106, 4018–4023 (2009).
Vroon, A., Heijnen, C.J. & Kavelaars, A. GRKs and arrestins: regulators of migration and inflammation. J. Leukoc. Biol. 80, 1214–1221 (2006).
Penela, P., Ribas, C. & Mayor, F. Jr. Mechanisms of regulation of the expression and function of G protein–coupled receptor kinases. Cell. Signal. 15, 973–981 (2003).
Schmitz, J. et al. IL-33, an interleukin-1–like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2–associated cytokines. Immunity 23, 479–490 (2005).
Chackerian, A.A. et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 179, 2551–2555 (2007).
Ali, S. et al. IL-1 receptor accessory protein is essential for IL-33–induced activation of T lymphocytes and mast cells. Proc. Natl. Acad. Sci. USA 104, 18660–18665 (2007).
Xu, D. et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 187, 787–794 (1998).
Moritz, D.R., Rodewald, H.R., Gheyselinck, J. & Klemenz, R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J. Immunol. 161, 4866–4874 (1998).
Coyle, A.J. et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2–mediated lung mucosal immune responses. J. Exp. Med. 190, 895–902 (1999).
Löhning, M. et al. T1/ST2 is preferentially expressed on murine TH2 cells, independent of interleukin 4, interleukin 5 and interleukin 10, and important for TH2 effector function. Proc. Natl. Acad. Sci. USA 95, 6930–6935 (1998).
Liew, F.Y., Xu, D., Brint, E.K. & O'Neill, L.A. Negative regulation of Toll-like receptor–mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Brint, E.K. et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 5, 373–379 (2004).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Plitas, G., Burt, B.M., Nguyen, H.M., Bamboat, Z.M. & DeMatteo, R.P. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 205, 1277–1283 (2008).
Alves-Filho, J.C., de Freitas, A., Russo, M. & Cunha, F.Q. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 34, 461–470 (2006).
Roger, T. et al. Protection from lethal Gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 106, 2348–2352 (2009).
Spiller, S. et al. TLR4-induced IFN-γ production increases TLR2 sensitivity and drives Gram-negative sepsis in mice. J. Exp. Med. 205, 1747–1754 (2008).
Angus, D.C. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001).
Hubbard, W.J. et al. Cecal ligation and puncture. Shock 24 Suppl 1, 52–57 (2005).
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006).
Cavaillon, J.M., Adib-Conquy, M., Fitting, C., Adrie, C. & Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 35, 535–544 (2003).
Brown, K.A. et al. Neutrophils in development of multiple organ failure in sepsis. Lancet 368, 157–169 (2006).
Cummings, C.J. et al. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J. Immunol. 162, 2341–2346 (1999).
Rios-Santos, F. et al. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase–derived nitric oxide. Am. J. Respir. Crit. Care Med. 175, 490–497 (2007).
Suzukawa, M. et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J. Immunol. 181, 5981–5989 (2008).
Mueller, S.G., White, J.R., Schraw, W.P., Lam, V. & Richmond, A. Ligand-induced desensitization of the human CXC chemokine receptor-2 is modulated by multiple serine residues in the carboxyl-terminal domain of the receptor. J. Biol. Chem. 272, 8207–8214 (1997).
Kurowska-Stolarska, M. et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477 (2009).
Arraes, S.M. et al. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood 108, 2906–2913 (2006).
Loniewski, K., Shi, Y., Pestka, J. & Parameswaran, N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol. Immunol. 45, 2312–2322 (2008).
Cherry, W.B., Yoon, J., Bartemes, K.R., Iijima, K. & Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 (2008).
Xu, D. et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. USA 105, 10913–10918 (2008).
Pushparaj, P.N. et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl. Acad. Sci. USA 106, 9773–9778 (2009).
Komai-Koma, M. et al. IL-33 is a chemoattractant for human TH2 cells. Eur. J. Immunol. 37, 2779–2786 (2007).
Townsend, M.J., Fallon, P.G., Matthews, D.J., Jolin, H.E. & McKenzie, A.N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 (2000).
Manley, M.O., O'Riordan, M.A., Levine, A.D. & Latifi, S.Q. Interleukin 10 extends the effectiveness of standard therapy during late sepsis with serum interleukin 6 levels predicting outcome. Shock 23, 521–526 (2005).
Levy, M.M. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31, 1250–1256 (2003).
Acknowledgements
This work was supported by the Wellcome Trust, the UK Medical Research Council, the UK and the EU (to F.Y.L.) and the State of São Paulo Research Foundation and National Research Council, Brazil (to F.Q.C.). Tlr4−/− and Myd88−/− mice were from S. Akira (University of Osaka).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 848 kb)
Rights and permissions
About this article
Cite this article
Alves-Filho, J., Sônego, F., Souto, F. et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 16, 708–712 (2010). https://doi.org/10.1038/nm.2156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2156
This article is cited by
-
Vascular smooth muscle cells in response to cholesterol crystals modulates inflammatory cytokines release and promotes neutrophil extracellular trap formation
Molecular Medicine (2024)
-
Role of IL-33-ST2 pathway in regulating inflammation: current evidence and future perspectives
Journal of Translational Medicine (2023)
-
Immune dysregulation in sepsis: experiences, lessons and perspectives
Cell Death Discovery (2023)
-
A comparative study of IL-33 and its receptor ST2 in a C57BL/6 J mouse model of pulmonary Cryptococcus neoformans infection
Medical Microbiology and Immunology (2023)
-
IL-33 induces histidine decarboxylase, especially in c-kit+ cells and mast cells, and roles of histamine include negative regulation of IL-33-induced eosinophilia
Inflammation Research (2023)