Abstract
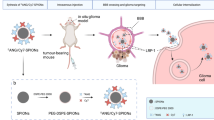
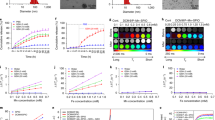
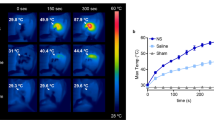
The difficulty in delineating brain tumor margins is a major obstacle in the path toward better outcomes for patients with brain tumors. Current imaging methods are often limited by inadequate sensitivity, specificity and spatial resolution. Here we show that a unique triple-modality magnetic resonance imaging–photoacoustic imaging–Raman imaging nanoparticle (termed here MPR nanoparticle) can accurately help delineate the margins of brain tumors in living mice both preoperatively and intraoperatively. The MPRs were detected by all three modalities with at least a picomolar sensitivity both in vitro and in living mice. Intravenous injection of MPRs into glioblastoma-bearing mice led to MPR accumulation and retention by the tumors, with no MPR accumulation in the surrounding healthy tissue, allowing for a noninvasive tumor delineation using all three modalities through the intact skull. Raman imaging allowed for guidance of intraoperative tumor resection, and a histological correlation validated that Raman imaging was accurately delineating the brain tumor margins. This new triple-modality–nanoparticle approach has promise for enabling more accurate brain tumor imaging and resection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bucci, M.K. et al. Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: results from a single center in the magnetic resonance imaging era. Cancer 101, 817–824 (2004).
Stupp, R. et al. Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist 11, 165–180 (2006).
Toms, S.A. et al. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Neurosurgery 57, 382–391, discussion 382–391 (2005).
Orringer, D.A. et al. The brain tumor window model: a combined cranial window and implanted glioma model for evaluating intraoperative contrast agents. Neurosurgery 66, 736–743 (2010).
Reinges, M.H. et al. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir. (Wien) 146, 369–377, discussion 377 (2004).
Lüdemann, L., Hamm, B. & Zimmer, C. Pharmacokinetic analysis of glioma compartments with dynamic Gd-DTPA-enhanced magnetic resonance imaging. Magn. Reson. Imaging 18, 1201–1214 (2000).
Knauth, M., Wirtz, C.R., Aras, N. & Sartor, K. Low-field interventional MRI in neurosurgery: finding the right dose of contrast medium. Neuroradiology 43, 254–258 (2001).
Knauth, M. et al. Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am. J. Neuroradiol. 20, 1547–1553 (1999).
Beljebbar, A., Dukic, S., Amharref, N. & Manfait, M. Ex vivo and in vivo diagnosis of C6 glioblastoma development by Raman spectroscopy coupled to a microprobe. Anal. Bioanal. Chem. 398, 477–487 (2010).
Ozawa, T. et al. Bromophenol blue staining of tumors in a rat glioma model. Neurosurgery 57, 1041–1047, discussion 1041–1047 (2005).
Shinoda, J. et al. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J. Neurosurg. 99, 597–603 (2003).
Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401 (2006).
de la Zerda, A. et al. A comparison between time domain and spectral imaging systems for imaging quantum dots in small living animals. Mol. Imaging Biol. 12, 500–508 (2010).
Kantelhardt, S.R. et al. Multiphoton excitation fluorescence microscopy of 5-aminolevulinic acid induced fluorescence in experimental gliomas. Lasers Surg. Med. 40, 273–281 (2008).
de la Zerda, A. et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 3, 557–562 (2008).
Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 3, 503–509 (2009).
Zavaleta, C.L., Kircher, M.F. & Gambhir, S.S. Raman's “effect” on molecular imaging. J. Nucl. Med. 52, 1839–1844 (2011).
Keren, S. et al. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 105, 5844–5849 (2008).
Zavaleta, C.L. et al. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Lett. 8, 2800–2805 (2008).
Zavaleta, C.L. et al. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl. Acad. Sci. USA 106, 13511–13516 (2009).
Adiseshaiah, P.P., Hall, J.B. & McNeil, S.E. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2, 99–112 (2010).
Tréhin, R. et al. Fluorescent nanoparticle uptake for brain tumor visualization. Neoplasia 8, 302–311 (2006).
Loening, A.M. & Gambhir, S.S. AMIDE: a free software tool for multimodality medical image analysis. Mol. Imaging 2, 131–137 (2003).
Vivanco, I. et al. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc. Natl. Acad. Sci. USA 107, 6459–6464 (2010).
Ermilov, S.A. et al. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 14, 024007 (2009).
Manohar, S. et al. Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics. Opt. Express 15, 12277–12285 (2007).
de la Zerda, A. et al. Photoacoustic ocular imaging. Opt. Lett. 35, 270–272 (2010).
de la Zerda, A. et al. Ultrahigh sensitivity carbon nanotube agents for photoacoustic molecular imaging in living mice. Nano Lett. 10, 2168–2172 (2010).
de la Zerda, A., Kim, J.W., Galanzha, E.I., Gambhir, S.S. & Zharov, V.P. Advanced contrast nanoagents for photoacoustic molecular imaging, cytometry, blood test and photothermal theranostics. Contrast Media Mol. Imaging 6, 346–369 (2011).
Zuin, S. et al. Weight of evidence approach for the relative hazard ranking of nanomaterials. Nanotoxicology 5, 445–458 (2011).
Kircher, M.F., Gambhir, S.S. & Grimm, J. Noninvasive cell-tracking methods. Nat. Rev. Clin. Oncol. 8, 677–688 (2011).
Thakor, A.S. et al. The fate and toxicity of Raman-active silica-gold nanoparticles in mice. Sci. Transl. Med. 3, 79ra33 (2011).
Thakor, A.S. et al. Oxidative stress mediates the effects of Raman-active gold nanoparticles in human cells. Small 7, 126–136 (2011).
Zavaleta, C.L. et al. Preclinical evaluation of Raman nanoparticle biodistribution for their potential use in clinical endoscopy imaging. Small 7, 2232–2240 (2011).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 (2000).
Koljenović, S. et al. Raman spectroscopic characterization of porcine brain tissue using a single fiber-optic probe. Anal. Chem. 79, 557–564 (2007).
Short, M.A. et al. Development and preliminary results of an endoscopic Raman probe for potential in vivo diagnosis of lung cancers. Opt. Lett. 33, 711–713 (2008).
Graves, E.E., Quon, A. & Loo, B.W. Jr. RT_Image: an open-source tool for investigating PET in radiation oncology. Technol. Cancer Res. Treat. 6, 111–121 (2007).
American National Standards Institute. American national standard for the safe use of lasers. in ANSI Standard Z136.1–2000 (ANSI, Inc., New York, 2000).
Acknowledgements
We thank M. Gozin for help with ICP-AES, L. Pisani for assistance with quantifying and acquiring magnetic resonance data, S.M. Korn and S. Bodapati for assistance in conducting the photoacoustic experiments, J. Rosenberg for assistance with the statistical analysis and the Memorial Sloan-Kettering Cancer Center Animal Imaging Core Facility (J. Koutcher and C.C. Le) for technical assistance. M.F.K. would like to thank R. Herfkens and the Stanford Department of Radiology for providing academic time to perform the study. We would like to acknowledge the following funding sources: National Cancer Institute grants CCNE U54 CA119367 (S.S.G.), CCNE U54 U54CA151459 (S.S.G.) and ICMIC P50 CA114747 (S.S.G.); The Ben and Catherine Ivy Foundation (S.S.G.); the Canary Foundation (S.S.G.); the Sir Peter Michael Foundation (S.S.G.); the Bio-X Graduate Student Fellowship (A.d.l.Z.); the Department of Defense Breast Cancer Research Program—Pre-doctoral Traineeship Award BC083014 (A.d.l.Z.) and the National Cancer Institute SMIS R25T Fellowship 5R25CA118681 (J.V.J.). The authors would also like to thank T.F. Massoud, D. Akin, H.E. Daldrup-Link, S. Bohndiek, S. Harmsen and J. Samii for critical review of the manuscript and B.T. Khuri-Yakub, S. Vaithilingam, O. Oralkan, E.E. Graves and H. Fan-Minogue for helpful discussions.
Author information
Authors and Affiliations
Contributions
M.F.K. co-initiated the project, designed the research, synthesized and characterized MPR nanoparticles, performed MRI, Raman, photoacoustic and histology experiments, analyzed data and wrote the manuscript. A.d.l.Z. modified the photoacoustic system, designed and performed photoacoustic experiments, analyzed data and wrote the manuscript. J.V.J. synthesized and characterized MPR nanoparticles. C.L.Z. designed, performed and analyzed Raman experiments and edited the paper. P.J.K. and R.S. performed and analyzed the electron microscopy experiments. K.P. performed immunohistochemistry. F.H. helped create three-dimensional renderings. E.M., M.F.K., K.P., R.H., C.C., C.W.B., I.K.M. and E.C.H. provided mouse models. S.S.G. co-initiated the project, designed the research, analyzed data, supervised and coordinated all investigators for the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14, Supplementary Discussion and Supplementary Methods (PDF 5246 kb)
Supplementary Video 1
Three-dimensional STEM rendering of MPR nanoparticles in U87MG tumor (AVI 9619 kb)
Rights and permissions
About this article
Cite this article
Kircher, M., de la Zerda, A., Jokerst, J. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med 18, 829–834 (2012). https://doi.org/10.1038/nm.2721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2721
This article is cited by
-
Radiolabeled nanomaterial for cancer diagnostics and therapeutics: principles and concepts
Cancer Nanotechnology (2023)
-
Omniparticle Contrast Agent for Multimodal Imaging: Synthesis and Characterization in an Animal Model
Molecular Imaging and Biology (2023)
-
Direct and quantitative assessments of near-infrared light attenuation and spectroscopic detection depth in biological tissues using surface-enhanced Raman scattering
Med-X (2023)
-
Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma
Journal of Nanobiotechnology (2022)