Abstract
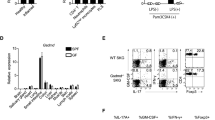
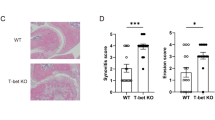
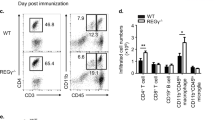
Regulatory T (Treg) cells suppress autoimmune disease, and impaired Treg cell function is associated with rheumatoid arthritis. Here we demonstrate that forkhead box P3 (FOXP3) transcriptional activity and, consequently, Treg cell suppressive function are regulated by phosphorylation at Ser418 in the C-terminal DNA-binding domain. In rheumatoid arthritis–derived Treg cells, the Ser418 site was specifically dephosphorylated by protein phosphatase 1 (PP1), whose expression and enzymatic activity were induced in the inflamed synovium by tumor necrosis factor α (TNF-α), leading to impaired Treg cell function. Moreover, TNF-α–induced Treg cell dysfunction correlated with increased numbers of interleukin-17 (IL-17)+ and interferon-γ (IFN-γ)+CD4+ T cells within the inflamed synovium in rheumatoid arthritis. Treatment with a TNF-α–specific antibody restored Treg cell function in subjects with rheumatoid arthritis, which was associated with decreased PP1 expression and increased FOXP3 phosphorylation in Treg cells. Thus, TNF-α controls the balance between Treg cells and pathogenic TH17 and TH1 cells in the synovium of individuals with rheumatoid arthritis through FOXP3 dephosphorylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Feldmann, M., Brennan, F.M. & Maini, R.N. Rheumatoid arthritis. Cell 85, 307–310 (1996).
Brennan, F.M. & McInnes, I.B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118, 3537–3545 (2008).
Suryaprasad, A.G. & Prindiville, T. The biology of TNF blockade. Autoimmun. Rev. 2, 346–357 (2003).
Ehrenstein, M.R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 (2004).
Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2, 364–371 (2002).
Ramesh, G. & Reeves, W.B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110, 835–842 (2002).
Ali, M. et al. Rheumatoid arthritis synovial T cells regulate transcription of several genes associated with antigen-induced anergy. J. Clin. Invest. 107, 519–528 (2001).
Puren, A.J., Fantuzzi, G., Gu, Y., Su, M.S. & Dinarello, C.A. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human blood mononuclear cells. J. Clin. Invest. 101, 711–721 (1998).
Popivanova, B.K. et al. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 118, 560–570 (2008).
Burstein, E. & Fearon, E.R. Colitis and cancer: a tale of inflammatory cells and their cytokines. J. Clin. Invest. 118, 464–467 (2008).
Wicovsky, A. et al. Tumor necrosis factor receptor-associated factor-1 enhances proinflammatory TNF receptor-2 signaling and modifies TNFR1-TNFR2 cooperation. Oncogene 28, 1769–1781 (2009).
Shevach, E.M. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 18, 423–449 (2000).
Costantino, C.M., Baecher-Allan, C. & Hafler, D.A. Multiple sclerosis and regulatory T cells. J. Clin. Immunol. 28, 697–706 (2008).
Riley, J.L., June, C.H. & Blazar, B.R. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30, 656–665 (2009).
Shevach, E.M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009).
Korn, T. et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13, 423–431 (2007).
Valencia, X. et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108, 253–261 (2006).
Ranganathan, P. Pharmacogenomics of tumor necrosis factor antagonists in rheumatoid arthritis. Pharmacogenomics 6, 481–490 (2005).
Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 241, 260–268 (2011).
Tao, R. et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13, 1299–1307 (2007).
Wang, L., Tao, R. & Hancock, W.W. Using histone deacetylase inhibitors to enhance Foxp3+ regulatory T-cell function and induce allograft tolerance. Immunol. Cell Biol. 87, 195–202 (2009).
Li, B. et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. USA 104, 4571–4576 (2007).
Samanta, A. et al. TGF-β and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc. Natl. Acad. Sci. USA 105, 14023–14027 (2008).
Putnam, A.L. et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58, 652–662 (2009).
Kim, J.Y. et al. Functional and genomic analyses of FOXP3-transduced Jurkat-T cells as regulatory T (Treg)-like cells. Biochem. Biophys. Res. Commun. 362, 44–50 (2007).
Lopes, J.E. et al. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J. Immunol. 177, 3133–3142 (2006).
Mihindukulasuriya, K.A., Zhou, G., Qin, J. & Tan, T.H. Protein phosphatase 4 interacts with and down-regulates insulin receptor substrate 4 following tumor necrosis factor-α stimulation. J. Biol. Chem. 279, 46588–46594 (2004).
Schett, G., Steiner, C.W., Xu, Q., Smolen, J.S. & Steiner, G. TNFα mediates susceptibility to heat-induced apoptosis by protein phosphatase-mediated inhibition of the HSF1/hsp70 stress response. Cell Death Differ. 10, 1126–1136 (2003).
Wu, Y. et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126, 375–387 (2006).
de Zoeten, E.F. et al. Foxp3 processing by proprotein convertases and control of regulatory T cell function. J. Biol. Chem. 284, 5709–5716 (2009).
Hollstein, M. & Hainaut, P. Massively regulated genes: the example of TP53. J. Pathol. 220, 164–173 (2010).
Huang, B., Yang, X.D., Lamb, A. & Chen, L.F. Posttranslational modifications of NF-κB: another layer of regulation for NF-κB signaling pathway. Cell Signal. 22, 1282–1290 (2010).
Chen, L.F. et al. NF-κB RelA phosphorylation regulates RelA acetylation. Mol. Cell Biol. 25, 7966–7975 (2005).
Nie, Y. et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat. Cell Biol. 11, 492–500 (2009).
Matsuzaki, H. et al. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl. Acad. Sci. USA 102, 11278–11283 (2005).
Zanin-Zhorov, A. et al. Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328, 372–376 (2010).
Pan, F. et al. Eos Mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 325, 1142–1146 (2009).
Acknowledgements
This work was partly supported by grants from National Natural Science Foundation of China (81072470) and the Shanghai Municipal Education Commission (J50207).
Author information
Authors and Affiliations
Contributions
H.N., Y.Z., R.L. and J.Z.Z. designed and discussed the study. H.N., Y.Z. and R.L. performed the majority of the experiments and analyzed the data. L.F., X.C. and B.W. performed cell culture and retroviral transduction. D.H. and L.X. recruited study participants and provided clinical samples. H.N., Y.Z., R.L., T.B.G., L.F., X.L., Y.E.C. and J.Z.Z. contributed to the writing of the paper. J.Z.Z. supervised the project and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Supplementary Tables 1–3 (PDF 809 kb)
Rights and permissions
About this article
Cite this article
Nie, H., Zheng, Y., Li, R. et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med 19, 322–328 (2013). https://doi.org/10.1038/nm.3085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3085
This article is cited by
-
The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases
Nature Reviews Immunology (2024)
-
CircGPRC5A enhances colorectal cancer progress by stabilizing PPP1CA and inducing YAP dephosphorylation
Journal of Experimental & Clinical Cancer Research (2023)
-
Conventional Tregs in treatment-naïve rheumatoid arthritis are deficient in suppressive function with an increase in percentage of CXCR3 and CCR6 expressing Tregs
Immunologic Research (2023)
-
FoxP3 expression by retinal pigment epithelial cells: transcription factor with potential relevance for the pathology of age-related macular degeneration
Journal of Neuroinflammation (2022)
-
Selective suppression of melanoma lacking IFN-γ pathway by JAK inhibition depends on T cells and host TNF signaling
Nature Communications (2022)