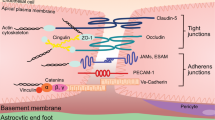
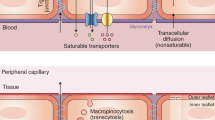
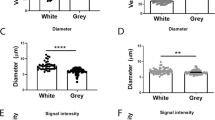
Abstract
The interface between the blood circulation and the neural tissue features unique characteristics that are encompassed by the term 'blood-brain barrier' (BBB). The main functions of this barrier, namely maintenance of brain homeostasis, regulation of influx and efflux transport, and protection from harm, are determined by its specialized multicellular structure. Every constituent cell type makes an indispensable contribution to the BBB's integrity. But if one member of the BBB fails, and as a result the barrier breaks down, there can be dramatic consequences and neuroinflammation and neurodegeneration can occur. In this Review, we highlight recently gained mechanistic insights into the development and maintenance of the BBB. We then discuss how BBB disruption can cause or contribute to neurological disease. Finally, we examine how this knowledge can be used to explore new possibilities for BBB repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abbott, N.J., Rönnbäck, L. & Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Ransohoff, R.M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635 (2012).
Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 72, 648–672 (2012).
Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 100, 158–173 (2007).
Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 100, 174–190 (2007).
Neuwelt, E.A. et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 12, 169–182 (2011).
Hallmann, R. et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 85, 979–1000 (2005).
Williams, K., Alvarez, X. & Lackner, A.A. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia 36, 156–164 (2001).
Daneman, R. et al. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE 5, e13741 (2010).
Shawahna, R. et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol. Pharm. 8, 1332–1341 (2011).
Lyck, R. et al. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J. Cereb. Blood Flow Metab. 29, 1491–1502 (2009).
Urich, E., Lazic, S.E., Molnos, J., Wells, I. & Freskgard, P.O. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS ONE 7, e38149 (2012).
Shalaby, F. et al. Failure of blood-island formation and vasculogenesis in Flk-1–deficient mice. Nature 376, 62–66 (1995).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996).
Raab, S. et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 91, 595–605 (2004).
Haigh, J.J. et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 262, 225–241 (2003).
Olsson, A.K., Dimberg, A., Kreuger, J. & Claesson-Welsh, L. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 (2006).
Daneman, R. et al. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641–646 (2009).
Logan, C.Y. & Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004).
Stenman, J.M. et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250 (2008).
Tam, S.J. et al. Death receptors DR6 and TROY regulate brain vascular development. Dev. Cell 22, 403–417 (2012).
Liebner, S. et al. Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 (2008).
Kuhnert, F. et al. Essential regulation of CNS angiogenesis by the orphan G protein–coupled receptor GPR124. Science 330, 985–989 (2010).
Anderson, K.D. et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein–coupled receptor. Proc. Natl. Acad. Sci. USA 108, 2807–2812 (2011).
Cullen, M. et al. GPR124, an orphan G protein–coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc. Natl. Acad. Sci. USA 108, 5759–5764 (2011).
Alvarez, J.I. et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 (2011).
Nagase, T., Nagase, M., Machida, M. & Fujita, T. Hedgehog signalling in vascular development. Angiogenesis 11, 71–77 (2008).
Pola, R. et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 7, 706–711 (2001).
Allt, G. & Lawrenson, J.G. Pericytes: cell biology and pathology. Cells Tissues Organs 169, 1–11 (2001).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Winkler, E.A., Bell, R.D. & Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Dore-Duffy, P. Pericytes: pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 14, 1581–1593 (2008).
Sá-Pereira, I., Brites, D. & Brito, M.A. Neurovascular unit: a focus on pericytes. Mol. Neurobiol. 45, 327–347 (2012).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Bell, R.D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Daneman, R., Zhou, L., Kebede, A.A. & Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Lindahl, P., Johansson, B.R., Leveen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B–deficient mice. Science 277, 242–245 (1997).
Hellström, M. et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 153, 543–553 (2001).
Hellström, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Li, F. et al. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev. Cell 20, 291–302 (2011).
Tallquist, M.D., French, W.J. & Soriano, P. Additive effects of PDGF receptor β signaling pathways in vascular smooth muscle cell development. PLoS Biol. 1, E52 (2003).
Lindblom, P. et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835–1840 (2003).
Gee, J.R. & Keller, J.N. Astrocytes: regulation of brain homeostasis via apolipoprotein E. Int. J. Biochem. Cell Biol. 37, 1145–1150 (2005).
Neuhaus, J. Orthogonal arrays of particles in astroglial cells: quantitative analysis of their density, size, and correlation with intramembranous particles. Glia 3, 241–251 (1990).
Stewart, P.A. & Wiley, M.J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev. Biol. 84, 183–192 (1981).
Hayashi, Y. et al. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 19, 13–26 (1997).
Sun, D., Lytle, C. & O'Donnell, M.E. IL-6 secreted by astroglial cells regulates Na-K-Cl cotransport in brain microvessel endothelial cells. Am. J. Physiol. 272, C1829–C1835 (1997).
Igarashi, Y. et al. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem. Biophys. Res. Commun. 261, 108–112 (1999).
Sobue, K. et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci. Res. 35, 155–164 (1999).
Lee, S.W. et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 9, 900–906 (2003).
Milsted, A., Barna, B.P., Ransohoff, R.M., Brosnihan, K.B. & Ferrario, C.M. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proc. Natl. Acad. Sci. USA 87, 5720–5723 (1990).
Wosik, K. et al. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J. Neurosci. 27, 9032–9042 (2007).
Hafezi-Moghadam, A., Thomas, K.L. & Wagner, D.D. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am. J. Physiol. Cell Physiol. 292, C1256–C1262 (2007).
Nishitsuji, K., Hosono, T., Nakamura, T., Bu, G. & Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 286, 17536–17542 (2011).
Bell, R.D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Deane, R. et al. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 118, 4002–4013 (2008).
Carvey, P.M., Hendey, B. & Monahan, A.J. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J. Neurochem. 111, 291–314 (2009).
Baeten, K.M. & Akassoglou, K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 71, 1018–1039 (2011).
Milner, R. & Campbell, I.L. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol. Cell. Neurosci. 20, 616–626 (2002).
Wang, J. & Milner, R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through α5β1 and αvβ3 integrins via MAP kinase signalling. J. Neurochem. 96, 148–159 (2006).
Osada, T. et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β1-integrins. J. Cereb. Blood Flow Metab. 31, 1972–1985 (2011).
Zhu, J. et al. β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129, 2891–2903 (2002).
McCarty, J.H. et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking αv integrins. Mol. Cell. Biol. 22, 7667–7677 (2002).
Cambier, S. et al. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am. J. Pathol. 166, 1883–1894 (2005).
Kostka, G. et al. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1–deficient mice. Mol. Cell. Biol. 21, 7025–7034 (2001).
Dong, L. et al. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab. Invest. 82, 1617–1630 (2002).
Pöschl, E. et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 (2004).
Smolich, B.D., McMahon, J.A., McMahon, A.P. & Papkoff, J. Wnt family proteins are secreted and associated with the cell surface. Mol. Biol. Cell 4, 1267–1275 (1993).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Liebner, S. & Plate, K.H. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J. Angiogenes. Res. 2, 1 (2010).
Akhmetshina, A. et al. Activation of canonical Wnt signalling is required for TGF-β–mediated fibrosis. Nat. Commun. 3, 735 (2012).
Handel, T.M., Johnson, Z., Crown, S.E., Lau, E.K. & Proudfoot, A.E. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu. Rev. Biochem. 74, 385–410 (2005).
Proudfoot, A.E. The biological relevance of chemokine-proteoglycan interactions. Biochem. Soc. Trans. 34, 422–426 (2006).
Shechter, R. & Schwartz, M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer 'if' but 'how'. J. Pathol. 229, 332–346 (2013).
Robberecht, W. Oxidative stress in amyotrophic lateral sclerosis. J. Neurol. 247, I1–I6 (2000).
van Horssen, J., Witte, M.E., Schreibelt, G. & de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 1812, 141–150 (2011).
Olmez, I. & Ozyurt, H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem. Int. 60, 208–212 (2012).
Davis, S.M. & Donnan, G.A. Secondary prevention after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 366, 1914–1922 (2012).
Suzuki, R., Yamaguchi, T., Kirino, T., Orzi, F. & Klatzo, I. The effects of 5-minute ischemia in Mongolian gerbils: I. Blood-brain barrier, cerebral blood flow, and local cerebral glucose utilization changes. Acta Neuropathol. 60, 207–216 (1983).
Pillai, D.R. et al. Cerebral ischemia-reperfusion injury in rats—A 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J. Cereb. Blood Flow Metab. 29, 1846–1855 (2009).
Pun, P.B., Lu, J. & Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 43, 348–364 (2009).
Montaner, J. et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 107, 598–603 (2003).
Gu, Y. et al. Caveolin-1 regulates nitric oxide–mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 120, 147–156 (2012).
Jiao, H., Wang, Z., Liu, Y., Wang, P. & Xue, Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood–brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 44, 130–139 (2011).
Bright, R. et al. Protein kinase C δ mediates cerebral reperfusion injury in vivo. J. Neurosci. 24, 6880–6888 (2004).
Chou, W.H. et al. Neutrophil protein kinase Cδ as a mediator of stroke-reperfusion injury. J. Clin. Invest. 114, 49–56 (2004).
Kunsch, C. & Medford, R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 85, 753–766 (1999).
Enzmann, G. et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol. 125, 395–412 (2013).
Madden, J.A. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology 79, S58–S62 (2012).
French, J.A. & Pedley, T.A. Clinical practice. Initial management of epilepsy. N. Engl. J. Med. 359, 166–176 (2008).
Fieschi, C., Lenzi, G.L., Zanette, E., Orzi, F. & Passero, S. Effects on EEG of the osmotic opening of the blood-brain barrier in rats. Life Sci. 27, 239–243 (1980).
Marchi, N. et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 48, 732–742 (2007).
Rapoport, S.I. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 20, 217–230 (2000).
Kofuji, P. & Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056 (2004).
Coulter, D.A. & Eid, T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 60, 1215–1226 (2012).
Cacheaux, L.P. et al. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J. Neurosci. 29, 8927–8935 (2009).
Ivens, S. et al. TGF-β receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130, 535–547 (2007).
Heinemann, U., Kaufer, D. & Friedman, A. Blood-brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia 60, 1251–1257 (2012).
Ravizza, T. et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 29, 142–160 (2008).
Morin-Brureau, M. et al. Epileptiform activity induces vascular remodeling and zonula occludens 1 downregulation in organotypic hippocampal cultures: role of VEGF signaling pathways. J. Neurosci. 31, 10677–10688 (2011).
Librizzi, L., Noe, F., Vezzani, A., de Curtis, M. & Ravizza, T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann. Neurol. 72, 82–90 (2012).
Fabene, P.F. et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 14, 1377–1383 (2008).
Palmer, A.M. The role of the blood-CNS barrier in CNS disorders and their treatment. Neurobiol. Dis. 37, 3–12 (2010).
Rowland, L.P. & Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 (2001).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Evans, M.C., Couch, Y., Sibson, N. & Turner, M.R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 53, 34–41 (2013).
Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Garbuzova-Davis, S. et al. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE 2, e1205 (2007).
Nicaise, C. et al. Impaired blood–brain and blood–spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 1301, 152–162 (2009).
Zhong, Z. et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 11, 420–422 (2008).
Miyazaki, K. et al. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J. Neurosci. Res. 89, 718–728 (2011).
Wingerchuk, D.M., Lennon, V.A., Lucchinetti, C.F., Pittock, S.J. & Weinshenker, B.G. The spectrum of neuromyelitis optica. Lancet Neurol. 6, 805–815 (2007).
Cree, B.A., Goodin, D.S. & Hauser, S.L. Neuromyelitis optica. Semin. Neurol. 22, 105–122 (2002).
Lennon, V.A. et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004).
Lennon, V.A., Kryzer, T.J., Pittock, S.J., Verkman, A.S. & Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Lucchinetti, C.F. et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125, 1450–1461 (2002).
Misu, T. et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130, 1224–1234 (2007).
Roemer, S.F. et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130, 1194–1205 (2007).
Takahashi, T. et al. Anti–aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 130, 1235–1243 (2007).
Kim, S.H. et al. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti–aquaporin-4 antibody levels. J. Clin. Neurol. 9, 36–42 (2013).
Cree, B.A. et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64, 1270–1272 (2005).
Hinson, S.R. et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 69, 2221–2231 (2007).
Haj-Yasein, N.N. et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl. Acad. Sci. USA 108, 17815–17820 (2011).
Hosokawa, T. et al. Increased serum matrix metalloproteinase-9 in neuromyelitis optica: implication of disruption of blood-brain barrier. J. Neuroimmunol. 236, 81–86 (2011).
Tomizawa, Y. et al. Blood-brain barrier disruption is more severe in neuromyelitis optica than in multiple sclerosis and correlates with clinical disability. J. Int. Med. Res. 40, 1483–1491 (2012).
Cayrol, R., Saikali, P. & Vincent, T. Effector functions of antiaquaporin-4 autoantibodies in neuromyelitis optica. Ann. NY Acad. Sci. 1173, 478–486 (2009).
Phuan, P.W., Ratelade, J., Rossi, A., Tradtrantip, L. & Verkman, A.S. Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J. Biol. Chem. 287, 13829–13839 (2012).
Saadoun, S. et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361 (2010).
Vincent, T. et al. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. J. Immunol. 181, 5730–5737 (2008).
Kinoshita, M. et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem. Biophys. Res. Commun. 386, 623–627 (2009).
Bradl, M. et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 66, 630–643 (2009).
Shimizu, F. et al. Sera from neuromyelitis optica patients disrupt the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 83, 288–297 (2012).
Rhen, T. & Cidlowski, J.A. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 (2005).
Miller, D.H. et al. High dose steroids in acute relapses of multiple sclerosis: MRI evidence for a possible mechanism of therapeutic effect. J. Neurol. Neurosurg. Psychiatry 55, 450–453 (1992).
Blecharz, K.G. et al. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult. Scler. 16, 293–302 (2010).
Förster, C., Kahles, T., Kietz, S. & Drenckhahn, D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J. Physiol. (Lond.) 580, 937–949 (2007).
Förster, C. et al. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. (Lond.) 586, 1937–1949 (2008).
Go, K.G. et al. Effect of steroids on brain lipocortin immunoreactivity. Acta Neurochir. Suppl. (Wien) 60, 101–103 (1994).
Cristante, E. et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc. Natl. Acad. Sci. USA 110, 832–841 (2013).
Argaw, A.T. et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 122, 2454–2468 (2012).
da Silva Meirelles, L., Caplan, A.I. & Nardi, N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299 (2008).
Auletta, J.J. et al. The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy 4, 529–547 (2012).
Zacharek, A. et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 27, 1684–1691 (2007).
Pfeiffer, F. et al. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 122, 601–614 (2011).
Sohet, F. & Daneman, R. Genetic mouse models to study blood-brain barrier development and function. Fluids Barriers CNS 10, 3 (2013).
Geldenhuys, W.J., Allen, D.D. & Bloomquist, J.R. Novel models for assessing blood-brain barrier drug permeation. Expert Opin. Drug Metab. Toxicol. 8, 647–653 (2012).
Dolghih, E. & Jacobson, M.P. Predicting efflux ratios and blood-brain barrier penetration from chemical structure: combining passive permeability with active efflux by P-glycoprotein. ACS Chem. Neurosci. 4, 361–367 (2013).
Lippmann, E.S. et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 30, 783–791 (2012).
Thanabalasundaram, G., El-Gindi, J., Lischper, M. & Galla, H.-J. Methods to assess pericyte-endothelial cell interactions in a coculture model. Methods Mol. Biol. 686, 379–399 (2011).
Abbott, N.J., Dolman, D.E., Drndarski, S. & Fredriksson, S.M. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol. Biol. 814, 415–430 (2012).
Vandenhaute, E. et al. Modelling the neurovascular unit and the blood-brain barrier with the unique function of pericytes. Curr. Neurovasc. Res. 8, 258–269 (2011).
Hatherell, K., Couraud, P.O., Romero, I.A., Weksler, B. & Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 199, 223–229 (2011).
Sumi, N. et al. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell. Mol. Neurobiol. 30, 247–253 (2010).
Xue, Q. et al. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int. J. Biol. Sci. 9, 174–189 (2013).
Man, S. et al. CXCL12-induced monocyte-endothelial interactions promote lymphocyte transmigration across an in vitro blood-brain barrier. Sci. Transl. Med. 4, 119ra114 (2012).
Cucullo, L., Hossain, M., Tierney, W. & Janigro, D. A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci. 14, 18 (2013).
Dohgu, S. et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-β production. Brain Res. 1038, 208–215 (2005).
Liebner, S., Czupalla, C.J. & Wolburg, H. Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 55, 467–476 (2011).
Cucullo, L., Hossain, M., Puvenna, V., Marchi, N. & Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 12, 40 (2011).
Gursoy-Ozdemir, Y., Yemisci, M. & Dalkara, T. Microvascular protection is essential for successful neuroprotection in stroke. J. Neurochem. 123 (suppl. 2), 2–11 (2012).
Janigro, D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia 53 (suppl. 1), 26–34 (2012).
Fabene, P.F., Laudanna, C. & Constantin, G. Leukocyte trafficking mechanisms in epilepsy. Mol. Immunol. 55, 100–104 (2013).
Sagare, A.P., Bell, R.D. & Zlokovic, B.V. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a011452 (2012).
Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Garbuzova-Davis, S. et al. Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res. 1398, 113–125 (2011).
Lu, M. & Hu, G. Targeting metabolic inflammation in Parkinson's disease: implications for prospective therapeutic strategies. Clin. Exp. Pharmacol. Physiol. 39, 577–585 (2012).
Bartels, A.L. et al. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson's disease, PSP and MSA. J. Neural Transm. 115, 1001–1009 (2008).
Engelhardt, B. & Ransohoff, R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 33, 579–589 (2012).
Kleinschmidt-DeMasters, B.K., Miravalle, A., Schowinsky, J., Corboy, J. & Vollmer, T. Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J. Neuropathol. Exp. Neurol. 71, 604–617 (2012).
Hinson, S.R., McKeon, A. & Lennon, V.A. Neurological autoimmunity targeting aquaporin-4. Neuroscience 168, 1009–1018 (2010).
Gowdie, P., Twilt, M. & Benseler, S.M. Primary and secondary central nervous system vasculitis. J. Child Neurol. 27, 1448–1459 (2012).
Hajj-Ali, R.A. & Calabrese, L.H. Primary angiitis of the central nervous system. Autoimmun. Rev. 12, 463–466 (2013).
Gilden, D., Cohrs, R.J., Mahalingam, R. & Nagel, M.A. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 8, 731–740 (2009).
Combes, V., Guillemin, G.J., Chan-Ling, T., Hunt, N.H. & Grau, G.E. The crossroads of neuroinflammation in infectious diseases: endothelial cells and astrocytes. Trends Parasitol. 28, 311–319 (2012).
Takeuchi, H., Matsuda, K., Kitai, R., Sato, K. & Kubota, T. Angiogenesis in primary central nervous system lymphoma (PCNSL). J. Neurooncol. 84, 141–145 (2007).
Wolburg, H., Noell, S., Fallier-Becker, P., Mack, A.F. & Wolburg-Buchholz, K. The disturbed blood-brain barrier in human glioblastoma. Mol. Aspects Med. 33, 579–589 (2012).
Bartynski, W.S. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am. J. Neuroradiol. 29, 1036–1042 (2008).
Chodobski, A., Zink, B.J. & Szmydynger-Chodobska, J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2, 492–516 (2011).
Sánchez-del-Rio, M. & Reuter, U. Migraine aura: new information on underlying mechanisms. Curr. Opin. Neurol. 17, 289–293 (2004).
Mogi, M. & Horiuchi, M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ. J. 75, 1042–1048 (2011).
Acknowledgements
R.M.R. is supported by US National Insititutes of Health grant R21 NS074820 and the Guthy Jackson Charitable Foundation. R.D. is supported by the Program for Breakthrough Biomedical Sciences and the American Heart Association. B.O. is supported by a two-year Research Fellowship from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Obermeier, B., Daneman, R. & Ransohoff, R. Development, maintenance and disruption of the blood-brain barrier. Nat Med 19, 1584–1596 (2013). https://doi.org/10.1038/nm.3407
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3407