Abstract
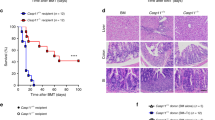
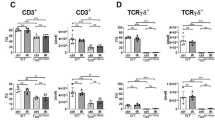
Acute graft-versus-host disease (GVHD) considerably limits wider usage of allogeneic hematopoietic cell transplantation (allo-HCT). Antigen-presenting cells and T cells are populations customarily associated with GVHD pathogenesis. Of note, neutrophils are the largest human white blood cell population. The cells cleave chemokines and produce reactive oxygen species, thereby promoting T cell activation1,2. Therefore, during an allogeneic immune response, neutrophils could amplify tissue damage caused by conditioning regimens. We analyzed neutrophil infiltration of the mouse ileum after allo-HCT by in vivo myeloperoxidase imaging and found that infiltration levels were dependent on the local microbial flora and were not detectable under germ-free conditions. Physical or genetic depletion of neutrophils reduced GVHD-related mortality. The contribution of neutrophils to GVHD severity required reactive oxygen species (ROS) because selective Cybb (encoding cytochrome b-245, beta polypeptide, also known as NOX2) deficiency in neutrophils impairing ROS production led to lower levels of tissue damage, GVHD-related mortality and effector phenotype T cells. Enhanced survival of Bcl-xL transgenic neutrophils increased GVHD severity. In contrast, when we transferred neutrophils lacking Toll-like receptor-2 (TLR2), TLR3, TLR4, TLR7 and TLR9, which are normally less strongly activated by translocating bacteria, into wild-type C57BL/6 mice, GVHD severity was reduced. In humans, severity of intestinal GVHD strongly correlated with levels of neutrophils present in GVHD lesions. This study describes a new potential role for neutrophils in the pathogenesis of GVHD in both mice and humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Tester, A.M. et al. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS ONE 2, e312 (2007).
Sena, L.A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Daley, J.M., Thomay, A.A., Connolly, M.D., Reichner, J.S. & Albina, J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 (2007).
Giroux, M. et al. SMAD3 prevents graft-versus-host disease by restraining TH1 differentiation and granulocyte-mediated tissue damage. Blood 117, 1734–1744 (2011).
Gross, S. et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 15, 455–461 (2009).
Yasuda, M. et al. Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1133–G1142 (2012).
Dale, D.C., Boxer, L. & Liles, W.C. The phagocytes: neutrophils and monocytes. Blood 112, 935–945 (2008).
Wang, G.G. et al. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293 (2006).
Gautam, S. et al. Survival and differentiation defects contribute to neutropenia in glucose-6-phosphatase-β (G6PC3) deficiency in a model of mouse neutrophil granulocyte differentiation. Cell Death Differ. 20, 1068–1079 (2013).
Robinson, K.M. et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 103, 15038–15043 (2006).
Ziegler, T.R. et al. Regulation of glutathione redox status in lung and liver by conditioning regimens and keratinocyte growth factor in murine allogeneic bone marrow transplantation. Transplantation 72, 1354–1362 (2001).
Zhang, Y., Joe, G., Hexner, E., Zhu, J. & Emerson, S.G. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J. Immunol. 174, 3051–3058 (2005).
Seger, R.A. et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood 100, 4344–4350 (2002).
Martinez, C.A. et al. Excellent survival after sibling or unrelated donor stem cell transplantation for chronic granulomatous disease. J. Allergy Clin. Immunol. 129, 176–183 (2012).
Reichardt, W. et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J. Immunol. 181, 4770–4779 (2008).
Jenq, R.R. et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 209, 903–911 (2012).
Fischer, M.A. et al. CD11b+, Ly6G+ cells produce type I interferon and exhibit tissue protective properties following peripheral virus infection. PLoS Pathog. 7, e1002374 (2011).
Dzhagalov, I., St. John, A. & He, Y.W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109, 1620–1626 (2007).
Kirschnek, S. et al. Molecular analysis of neutrophil spontaneous apoptosis reveals a strong role for the pro-apoptotic BH3-only protein Noxa. Cell Death Differ. 18, 1805–1814 (2011).
Koedel, U. et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 5, e1000461 (2009).
Li, H. et al. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J. Immunol. 186, 230–241 (2011).
Luo, H.R. & Loison, F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am. J. Hematol. 83, 288–295 (2008).
Hill, G.R. et al. Differential roles of IL-1 and TNF-α on graft-versus-host disease and graft versus leukemia. J. Clin. Invest. 104, 459–467 (1999).
Jankovic, D. et al. The Nlrp3-inflammasome regulates acute graft-versus-host disease. J. Exp. Med. 210, 1899–1910 (2013).
Forlenza, M. et al. Differential contribution of neutrophilic granulocytes and macrophages to nitrosative stress in a host-parasite animal model. Mol. Immunol. 45, 3178–3189 (2008).
Cooke, K.R. et al. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Invest. 102, 1882–1891 (1998).
Socié, G. et al. Prognostic value of apoptotic cells and infiltrating neutrophils in graft-versus-host disease of the gastrointestinal tract in humans: TNF and Fas expression. Blood 103, 50–57 (2004).
Leonhardt, F. et al. Inflammatory neovascularization during graft-versus-host disease is regulated by αv integrin and miR-100. Blood 121, 3307–3318 (2013).
Leonhardt, F. et al. Spleen tyrosine kinase (Syk) is a potent target for GVHD prevention at different cellular levels. Leukemia 26, 1617–1629 (2012).
Wilhelm, K. et al. Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nat. Med. 16, 1434–1438 (2010).
Sitia, G. et al. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 99, 13717–13722 (2002).
Lerner, K.G. et al. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant. Proc. 6, 367–371 (1974).
Leonhardt, F. et al. Inflammatory neovascularization during graft-versus-host disease is regulated by αv integrin and miR-100. Blood 121, 3307–3318 (2013).
Zeiser, R. et al. Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft versus host disease. Blood 109, 2225–2233 (2007).
Kaplan, D.H. et al. Target antigens determine graft-versus-host disease phenotype. J. Immunol. 173, 5467–5475 (2004).
Sadeghi, B. et al. GVHD after chemotherapy conditioning in allogeneic transplanted mice. Bone Marrow Transplant. 42, 807–818 (2008).
Turner, B.E. et al. Reduced intensity conditioning for allogeneic hematopoietic stem-cell transplant determines the kinetics of acute graft-versus-host disease. Transplantation 86, 968–976 (2008).
Wolf, P. et al. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate 70, 562–569 (2010).
Zeiser, R. et al. Differential impact of mTOR inhibition on CD4+CD25+Foxp3+ regulatory T cells as compared to conventional CD4+ T cells. Blood 111, 453–462 (2008).
Gross, S. et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 15, 455–461 (2009).
Wilhelm, K. et al. Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nat. Med. 16, 1434–1438 (2010).
Reichardt, W. et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J. Immunol. 181, 4770–4779 (2008).
Hoerr, V. et al. Bacteria tracking by in vivo magnetic resonance imaging. BMC Biol. 11, 63 (2013).
Haacke, E.M. et al. Susceptibility weighted imaging (SWI). Magn. Reson. Med. 52, 612–618 (2004).
Wang, G.G. et al. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293 (2006).
Weigmann, B. et al. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 (2007).
Irizarry, R.A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300 (1995).
Davies, J.A., Anderson, G.K., Beveridge, T.J. & Clark, H.C. Chemical mechanism of the gram stain and synthesis of a new electron-opaque marker for electron microscopy which replaces the iodine mordant of the stain. J. Bacteriol. 156, 837–845 (1983).
Robinson, K.M. et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 103, 15038–15043 (2006).
Acknowledgements
This study was supported by the Deutsche Jose Carreras Leukämie Stiftung (DJCLS grant # R12/11), Deutsche Forschungsgemeinschaft (DFG), Germany, Heisenberg Professorship to R.Z. (DFG ZE 872/3-1) and DFG individual grant to R.Z. (DFG ZE 872/1-2), in part by SFB 850 (to R.Z.), Excellence Initiative of the German Research Foundation (GSC-4, Spemann Graduate School to R.Z. and G.H. and BIOSS II, project no. B13 to R.Z.), Deutsche Krebshilfe (to R.Z.), and Marie Curie FP7-IRG268390 (to A.T.) and also in part by the IDEA 2012 award (LSU Health Sciences Center, to G.C.H.). A.M. was funded by the European Research Council and the Wellcome Trust. H.H. was supported by the American Lebanese Syrian Associated Charities (ALSAC). We thank M. Follo for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
L.S., L.G., S.P., F.J.K, S.V.R., A.T., S.G., M.F., F.L., A.S. and K.H. helped design the experiments and performed experiments. S.F.M., R.M., J.F., P.H., M.P., A.M., F.D.v.L. and J.D. helped design the experiments, provided essential reagents and discussed the data. O.S. helped collect patient samples and provided clinical data, H.H. helped design experiments with HoxB8 cells and discussed data. W.R. and N.B. performed and analyzed magnetic resonance imaging experiments. P.W. performed anti-Ly6G antibody measurement in serum of mice, analyzed data and helped write the manuscript. A.S.-G. analyzed GVHD histopathology and neutrophil infiltration. D.P. performed and analyzed microarray experiments. G.C.H., G.H. and R.Z. developed the overall concept, supervised the experiments, discussed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1 and 2 (PDF 4939 kb)
Rights and permissions
About this article
Cite this article
Schwab, L., Goroncy, L., Palaniyandi, S. et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med 20, 648–654 (2014). https://doi.org/10.1038/nm.3517
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3517
This article is cited by
-
The transplant rejection response involves neutrophil and macrophage adhesion-mediated trogocytosis and is regulated by NFATc3
Cell Death & Disease (2024)
-
ROCK1/2 signaling contributes to corticosteroid-refractory acute graft-versus-host disease
Nature Communications (2024)
-
Enforced mesenchymal stem cell tissue colonization counteracts immunopathology
npj Regenerative Medicine (2022)
-
Acute graft-versus-host disease increase risk and accuracy in prediction model of transplantation-associated thrombotic microangiopathy in patients with myelodysplastic syndrome
Annals of Hematology (2022)
-
Anti-osteosarcoma effect of antiserum against cross antigen TPD52 between osteosarcoma and Trichinella spiralis
Parasites & Vectors (2021)