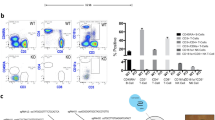
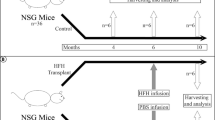
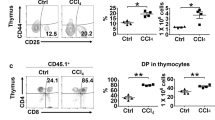
Abstract
Liver repopulation with bone marrow–derived hepatocytes (BMHs) can cure the genetic liver disease fumarylacetoacetate hydrolase (Fah) deficiency1. BMHs emerge from fusion between donor bone marrow–derived cells and host hepatocytes2. To use such in vivo cell fusion efficiently for therapy requires knowing the nature of the hematopoietic cells that fuse with hepatocytes. Here we show that the transplantation into Fah−/− mice of hematopoietic stem cells (HSCs) from lymphocyte-deficient Rag1−/− mice, lineage-committed granulocyte-macrophage progenitors (GMPs) or bone marrow–derived macrophages (BMMs) results in the robust production of BMHs. These results provide direct evidence that committed myelomonocytic cells such as macrophages can produce functional epithelial cells by in vivo fusion. Because stable bone marrow engraftment or HSCs are not required for this process, macrophages or their highly proliferative progenitors provide potential for targeted and well-tolerated cell therapy aimed at organ regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lagasse, E. et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6, 1229–1234 (2000).
Wang, X. et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 (2003).
Ferrari, G. et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528–1530 (1998).
Jackson, K.A. et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 107, 1395–402 (2001).
Mezey, E., Chandross, K.J., Harta, G., Maki, R.A. & McKercher, S.R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290, 1779–1782 (2000).
Petersen, B.E. et al. Bone marrow as a potential source of hepatic oval cells. Science 284, 1168–1170 (1999).
Alison, M.R. et al. Hepatocytes from non-hepatic adult stem cells. Nature 406, 257 (2000).
Krause, D.S. et al. Multi-organ, multi-lineage engraftment by a single bone marrow–derived stem cell. Cell 105, 369–377 (2001).
Vassilopoulos, G., Wang, P.R. & Russell, D.W. Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901–904 (2003).
Alvarez-Dolado, M. et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973 (2003).
Nygren, J.M. et al. Bone marrow–derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10, 494–501 (2004).
Weimann, J.M., Johansson, C.B., Trejo, A. & Blau, H.M. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 5, 959–966 (2003).
Overturf, K. et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 12, 266–273 (1996).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–77 (1992).
Bailey, A.S. et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 103, 13–19 (2004).
Wang, X. et al. Kinetics of liver repopulation after bone marrow transplantation. Am. J. Pathol. 161, 565–574 (2002).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Na Nakorn, T., Traver, D., Weissman, I.L. & Akashi, K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 109, 1579–1585 (2002).
BitMansour, A. et al. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood 100, 4660–4667 (2002).
Kondo, M., Weissman, I.L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Cozzio, A. et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 17, 3029–3035 (2003).
Friedrich, G. & Soriano, P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 (1991).
Kennedy, D.W. & Abkowitz, J.L. Mature monocytic cells enter tissues and engraft. Proc. Natl. Acad. Sci. USA 95, 14944–14949 (1998).
Freeman, B.J. et al. Behavior and therapeutic efficacy of β-glucuronidase-positive mononuclear phagocytes in a murine model of mucopolysaccharidosis type VII. Blood 94, 2142–2150 (1999).
Geissmann, F., Jung, S. & Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Van Rooijen, N. & Sanders, A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 23, 1239–1243 (1996).
Camargo, F.D., Green, R., Capetenaki, Y., Jackson, K.A. & Goodell, M.A. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med. 9, 1520–1527 (2003).
Jorquera, R. & Tanguay, R.M. Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum. Mol. Genet. 10, 1741–1752 (2001).
van de Rijn, M., Heimfeld, S., Spangrude, G.J. & Weissman, I.L. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc. Natl. Acad. Sci. USA 86, 4634–4638 (1989).
Grompe, M. et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 7, 2298–2307 (1993).
Acknowledgements
We thank C. Baumann, A. Major, G. Faulkner, C. Montgomery, A. Snyder, M. Al-Dhalimy and E. Speel for their work in support of this study, which was funded by US National Institutes of Health grants (RO1-DK067636 to M.G. and RO1-HL69133 to W.H.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Hematopoietic repopulation from transplanted GMP. (PDF 155 kb)
Supplementary Fig. 2
Isotype controls for GMP sorting (PDF 152 kb)
Rights and permissions
About this article
Cite this article
Willenbring, H., Bailey, A., Foster, M. et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med 10, 744–748 (2004). https://doi.org/10.1038/nm1062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1062
This article is cited by
-
Intrinsic signalling factors associated with cancer cell-cell fusion
Cell Communication and Signaling (2023)
-
Mesenchymal stromal cells: promising treatment for liver cirrhosis
Stem Cell Research & Therapy (2022)
-
Hepatocyte generation in liver homeostasis, repair, and regeneration
Cell Regeneration (2022)
-
Efficient hepatic differentiation and regeneration potential under xeno-free conditions using mass-producible amnion-derived mesenchymal stem cells
Stem Cell Research & Therapy (2021)
-
Donor Recipient Chimeric Cells Induce Chimerism and Extend Survival of Vascularized Composite Allografts
Archivum Immunologiae et Therapiae Experimentalis (2021)