Abstract
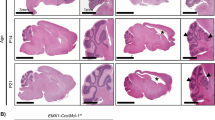
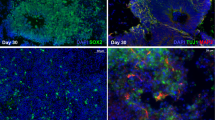
Vanishing white matter disease (VWM) is a heritable leukodystrophy linked to mutations in translation initiation factor 2B (eIF2B). Although the clinical course of this disease has been relatively well described, the cellular consequences of EIF2B mutations on neural cells are unknown. Here we have established cell cultures from the brain of an individual with VWM carrying mutations in subunit 5 of eIF2B (encoded by EIF2B5). Despite the extensive demyelination apparent in this VWM patient, normal-appearing oligodendrocytes were readily generated in vitro. In contrast, few GFAP-expressing (GFAP+) astrocytes were present in primary cultures, induction of astrocytes was severely compromised, and the few astrocytes generated showed abnormal morphologies and antigenic phenotypes. Lesions in vivo also lacked GFAP+ astrocytes. RNAi targeting of EIF2B5 severely compromised the induction of GFAP+ cells from normal human glial progenitors. This raises the possibility that a deficiency in astrocyte function may contribute to the loss of white matter in VWM leukodystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
van der Knaap, M.S. et al. A new leukoencephalopathy with vanishing white matter. Neurology 48, 845–855 (1997).
Schiffmann, R. et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann. Neurol. 35, 331–340 (1994).
Hanefeld, F. et al. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics 24, 244–248 (1993).
Fogli, A. et al. Cree leukoencephalopathy and CACH/VWM disease are allelic at the EIF2B5 locus. Ann. Neurol. 52, 506–510 (2002).
Fogli, A. et al. A severe variant of childhood ataxia with central hypomyelination/vanishing white matter leukoencephalopathy related to EIF21B5 mutation. Neurology 59, 1966–1968 (2002).
Leegwater, P.A. et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat. Genet. 29, 383–388 (2001).
van der Knaap, M.S. et al. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann. Neurol. 51, 264–270 (2002).
Palmer, T.D. et al. Cell culture. Progenitor cells from human brain after death. Nature 411, 42–43 (2001).
Westerlund, U. et al. Stem cells from the adult human brain develop into functional neurons in culture. Exp. Cell. Res. 289, 378–383 (2003).
Schwartz, P.H. et al. Isolation and characterization of neural progenitor cells from post–mortem human cortex. J. Neurosci. Res. 74, 838–851 (2003).
Hertz, L., Peng, L. & Lai, J.C. Functional studies in cultured astrocytes. Methods 16, 293–310 (1998).
Yong, V.W., Kim, S.U., Kim, M.W. & Shin, D.H. Growth factors for human glial cells in culture. Glia 1, 113–123 (1988).
Raff, M.C., Abney, E.R., Cohen, J., Lindsay, R. & Noble, M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J. Neurosci. 3, 1289–1300 (1983).
Kretzschmar, M., Liu, F., Hata, A., Doody, J. & Massague, J. The TGF–beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 11, 984–995 (1997).
Wong, K. et al. Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol. (Berl.) 100, 635–646. (2000).
Francalanci, P. et al. Fatal infantile leukodystrophy: a severe variant of CACH/VWM syndrome, allelic to chromosome 3q27. Neurology 57, 265–270 (2001).
Dietrich, J., Noble, M. & Mayer-Proschel, M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia 40, 65–77 (2002).
Sui, G. et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 5515–5520 (2002).
Gomez, E. & Pavitt, G.D. Identification of domains and residues within the epsilon subunit of eukaryotic translation initiation factor 2B (eIF2Bepsilon) required for guanine nucleotide exchange reveals a novel activation function promoted by eIF2B complex formation. Mol. Cell. Biol. 20, 3965–3976 (2000).
Anthony, T.G., Fabian, J.R., Kimball, S.R. & Jefferson, L.S. Identification of domains within the epsilon–subunit of the translation initiation factor eIF2B that are necessary for guanine nucleotide exchange activity and eIF2B holoprotein formation. Biochim. Biophys. Acta 1492, 56–62 (2000).
Asano, K., Krishnamoorthy, T., Phan, L., Pavitt, G.D. & Hinnebusch, A.G. Conserved bipartite motifs in yeast eIF5 and eIF2Bepsilon, GTPase–activating and GDP–GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J. 18, 1673–1688 (1999).
Fogli, A. et al. Decreased guanine nucleotide exchange factor activity in eIF2B–mutated patients. Eur. J. Hum. Genet. 12, 561–566 (2004).
van der Knaap, M.S. et al. Phenotypic variation in leukoencephalopathy with vanishing white matter. Neurology 51, 540–547 (1998).
Van Haren, K., van der Voorn, J.P., Peterson, D.R., van der Knaap, M.S. & Powers, J.M. The life and death of oligodendrocytes in vanishing white matter disease. J. Neuropathol. Exp. Neurol. 63, 618–630 (2004).
Pekny, M. et al. Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J. 14, 1590–1598 (1995).
Gomi, H. et al. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 14, 29–41 (1995).
McCall, M.A. et al. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc. Natl. Acad. Sci. USA 93, 6361–6366 (1996).
Liedtke, W. et al. GFAP is necessary for the integrity of CNS white matter architecture and long–term maintenance of myelination. Neuron 17, 607–615 (1996).
Nawashiro, H., Messing, A., Azzam, N. & Brenner, M. Mice lacking GFAP are hypersensitive to traumatic cerebrospinal injury. Neuroreport 9, 1691–1696 (1998).
Nawashiro, H., Brenner, M., Fukui, S., Shima, K. & Hallenbeck, J.M. High susceptibility to cerebral ischemia in GFAP–null mice. J Cereb. Blood Flow Metab. 20, 1040–1044 (2000).
Pekny, M. et al. The impact of genetic removal of GFAP and/or vimentin on glutamine levels and transport of glucose and ascorbate in astrocytes. Neurochem. Res. 24, 1357–1362 (1999).
Xu, K., Malouf, A.T., Messing, A. & Silver, J. Glial fibrillary acidic protein is necessary for mature astrocytes to react to beta–amyloid. Glia 25, 390–403 (1999).
Brenner, M. et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27, 117–120 (2001).
Gorospe, J.R. et al. Molecular findings in symptomatic and pre–symptomatic Alexander disease patients. Neurology 58, 1494–1500. (2002).
Rodriguez, D. et al. Infantile Alexander disease: spectrum of GFAP mutations and genotype–phenotype correlation. Am. J. Hum. Genet. 69, 1134–1140 (2001).
Nedergaard, M., Ransom, B. & Goldman, S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530 (2003).
Windrem, M.S. et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 10, 93–97 (2004).
Han, S.S., Liu, Y., Tyler–Polsz, C., Rao, M.S. & Fischer, I. Transplantation of glial–restricted precursor cells into the adult spinal cord: survival, glial–specific differentiation, and preferential migration in white matter. Glia 45, 1–16 (2004).
Herrera, J. et al. Embryonic–derived glial–restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Exp. Neurol. 171, 11–21 (2001).
van der Knaap, M.S. Magnetic resonance in childhood white–matter disorders. Dev. Med. Child Neurol. 43, 705–712 (2001).
Bottenstein, J.E. & Sato, G.H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA 76, 514–517 (1979).
Mayer-Proschel, M., Kalyani, A.J., Mujtaba, T. & Rao, M.S. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron 19, 773–785 (1997).
Rao, M.S., Noble, M. & Mayer-Proschel, M. A tripotential glial precursor cell is present in the developing spinal cord. Proc. Natl. Acad. Sci. USA 95, 3996–4001 (1998).
Rao, M.S. & Mayer–Proschel, M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev. Biol. 188, 48–63. (1997).
Gregori, N., Proschel, C., Noble, M. & Mayer–Proschel, M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte–type–2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J. Neurosci. 22, 248–256 (2002).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Gomez, E., Mohammad, S.S. & Pavitt, G.D. Characterization of the minimal catalytic domain within eIF2B: the guanine-nucleotide exchange factor for translation initiation. EMBO J. 21, 5292–5301 (2002).
Acknowledgements
We thank J. Powers for providing support and access to patient samples, H. Land for discussions and L. Silver for technical assistance. This work was supported by grants from the Children's Neurobiological Solutions Foundation (to M.M.-P. and M.N.), the Multiple Sclerosis Society of Canada (to M.M.-P. and M.N.), and the US National Institutes of Health (NS047411 to C.P., NS42820 to M.M.-P. and C.P., and HD39702 to M.N.). J.D. is a fellow of the Wilmot Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Paucity of GFAP+ astrocytes in demyelinating lesions of a CACH/VWM patient with known mutations in the translation initation factor subunit eIF2B5. (PDF 260 kb)
Supplementary Fig. 2
CACH/VWM disease cells are responsive to BMP-4. (PDF 207 kb)
Supplementary Fig. 3
Comparison of GFAP expression in cerebral white matter sections derived from patients with different underlying neurological diseases affecting white matter tracts. (PDF 327 kb)
Supplementary Fig. 4
RNAi inhibition of eIF2B5 expression. (PDF 89 kb)
Supplementary Table 1
Oligodeoxynucleotides used for the construction of RNAi expression plasmids targeting different regions of human eIF2B5 (PDF 21 kb)
Rights and permissions
About this article
Cite this article
Dietrich, J., Lacagnina, M., Gass, D. et al. EIF2B5 mutations compromise GFAP+ astrocyte generation in vanishing white matter leukodystrophy. Nat Med 11, 277–283 (2005). https://doi.org/10.1038/nm1195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1195
This article is cited by
-
Secretomics Alterations and Astrocyte Dysfunction in Human iPSC of Leukoencephalopathy with Vanishing White Matter
Neurochemical Research (2022)
-
Foetal onset of EIF2B related disorder in two siblings: cerebellar hypoplasia with absent Bergmann glia and severe hypomyelination
Acta Neuropathologica Communications (2020)
-
Eukaryotic translation initiation factors as promising targets in cancer therapy
Cell Communication and Signaling (2020)
-
eIF2B Mutations Cause Mitochondrial Malfunction in Oligodendrocytes
NeuroMolecular Medicine (2019)
-
Translation deregulation in human disease
Nature Reviews Molecular Cell Biology (2018)