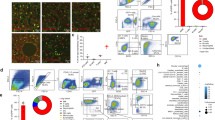
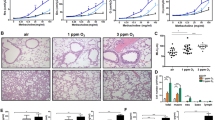
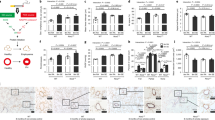
Abstract
Alveolar cell apoptosis is involved in the pathogenesis of emphysema, a prevalent disease primarily caused by cigarette smoking. We report that ceramide, a second messenger lipid, is a crucial mediator of alveolar destruction in emphysema. Inhibition of enzymes controlling de novo ceramide synthesis prevented alveolar cell apoptosis, oxidative stress and emphysema caused by blockade of the vascular endothelial growth factor (VEGF) receptors in both rats and mice. Emphysema was reproduced with intratracheal instillation of ceramide in naive mice. Excessive ceramide triggers a feed-forward mechanism mediated by activation of secretory acid sphingomyelinase, as suggested by experiments with neutralizing ceramide antibody in mice and with acid sphingomyelinase–deficient fibroblasts. Concomitant augmentation of signaling initiated by a prosurvival metabolite, sphingosine-1-phosphate, prevented lung apoptosis, implying that a balance between ceramide and sphingosine-1-phosphate is required for maintenance of alveolar septal integrity. Finally, increased lung ceramides in individuals with smoking-induced emphysema suggests that ceramide upregulation may be a crucial pathogenic element and a promising target in this disease that currently lacks effective therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis 132, 182–5. (1985).
Shapiro, S.D. Vascular atrophy and VEGFR-2 signaling: old theories of pulmonary emphysema meet new data. J. Clin. Invest. 106, 1309–1310 (2000).
Kasahara, Y. et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Invest. 106, 1311–1319 (2000).
Tuder, R.M., Petrache, I., Elias, J.A., Voelkel, N.F. & Henson, P.M. Apoptosis and emphysema: the missing link. Am. J. Respir. Cell Mol. Biol. 28, 551–554 (2003).
Tuder, R.M. et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am. J. Respir. Cell Mol. Biol. 29, 88–97 (2003).
Rangasamy, T. et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 114, 1248–1259 (2004).
Hannun, Y.A. & Obeid, L.M. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 (2002).
Reunanen, N. et al. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J. Biol. Chem. 273, 5137–5145 (1998).
Hannun, Y.A. & Luberto, C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 10, 73–80 (2000).
Kolesnick, R. & Fuks, Z. Radiation and ceramide-induced apoptosis. Oncogene 22, 5897–5906 (2003).
Luberto, C., Kraveka, J.M. & Hannun, Y.A. Ceramide regulation of apoptosis versus differentiation: a walk on a fine line. Lessons from neurobiology. Neurochem. Res. 27, 609–617 (2002).
Zhang, Y. et al. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell 89, 63–72 (1997).
Chalfant, C.E. et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J. Biol. Chem. 277, 12587–12595 (2002).
Heinrich, M. et al. Ceramide as an activator lipid of cathepsin D. Adv. Exp. Med. Biol. 477, 305–315 (2000).
Gulbins, E. & Grassme, H. Ceramide and cell death receptor clustering. Biochim. Biophys. Acta 1585, 139–145 (2002).
Siskind, L.J., Kolesnick, R.N. & Colombini, M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277, 26796–26803 (2002).
Goggel, R. et al. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat. Med. 10, 155–160 (2004).
Cuvillier, O. et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803 (1996).
Payne, S.G., Milstien, S. & Spiegel, S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 531, 54–57 (2002).
Morita, Y. & Tilly, J.L. Sphingolipid regulation of female gonadal cell apoptosis. Ann. NY Acad. Sci. 905, 209–220 (2000).
Kasahara, Y. et al. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 163, 737–744 (2001).
Norred, W.P., Voss, K.A., Riley, R.T. & Plattner, R.D. Fumonisin toxicity and metabolism studies at the USDA. Fumonisin toxicity and metabolism. Adv. Exp. Med. Biol. 392, 225–236 (1996).
Peng, X. et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 169, 1245–1251 (2004).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Ravid, T. et al. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am. J. Physiol. Lung Cell Mol. Physiol. 284, L1082–L1092 (2003).
Hautamaki, R.D., Kobayashi, D.K., Senior, R.M. & Shapiro, S.D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004 (1997).
Ogretmen, B. et al. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 277, 12960–12969 (2002).
Petrache, I. et al. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J. 17, 407–416 (2003).
Soler, N. et al. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur. Respir. J. 14, 1015–1022 (1999).
Slowik, M.R., De Luca, L.G., Min, W. & Pober, J.S. Ceramide is not a signal for tumor necrosis factor-induced gene expression but does cause programmed cell death in human vascular endothelial cells. Circ. Res. 79, 736–747 (1996).
Kroesen, B.J. et al. BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J. Biol. Chem. 278, 14723–14731 (2003).
Haimovitz-Friedman, A. et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J. Exp. Med. 186, 1831–1841 (1997).
Taraseviciene-Stewart, L. et al. An animal model of autoimmune emphysema. Am. J. Respir. Crit. Care Med. 171, 734–742 (2005).
Pfeiffer, A. et al. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31, 3153–3164 (2001).
Kolesnick, R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Invest. 110, 3–8 (2002).
Chavakis, E. & Dimmeler, S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler. Thromb. Vasc. Biol. 22, 887–893 (2002).
Pautz, A. et al. Cross-talk between nitric oxide and superoxide determines ceramide formation and apoptosis in glomerular cells. Kidney Int. 61, 790–796 (2002).
Lavrentiadou, S.N. et al. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am. J. Respir. Cell Mol. Biol. 25, 676–684 (2001).
Horinouchi, K. et al. Acid sphingomyelinase deficient mice: a model of types A and B Niemann- Pick disease. Nat. Genet. 10, 288–293 (1995).
Otterbach, B. & Stoffel, W. Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann-Pick disease). Cell 81, 1053–1061 (1995).
Ikegami, M., Dhami, R. & Schuchman, E.H. Alveolar lipoproteinosis in an acid sphingomyelinase-deficient mouse model of Niemann-Pick disease. Am. J. Physiol. Lung Cell Mol. Physiol. 284, L518–L525 (2003).
Fletcher, C. & Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1, 1645–1648 (1977).
Tilly, J.L. & Kolesnick, R.N. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim. Biophys. Acta 1585, 135–138 (2002).
Tuder, R.M., Wood, K., Taraseviciene, L., Flores, S.C. & Voekel, N.F. Cigarette smoke extract decreases the expression of vascular endothelial growth factor by cultured cells and triggers apoptosis of pulmonary endothelial cells. Chest 117, 241S–242S (2000).
Bligh, E.G. & Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37, 911–917 (1959).
Dobrowsky, R.T. & Kolesnick, R.N. Analysis of sphingomyelin and ceramide levels and the enzymes regulating their metabolism in response to cell stress. Methods Cell Biol. 66, 135–165 (2001).
Perry, D.K., Bielawska, A. & Hannun, Y.A. Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol. 312, 22–31 (2000).
Tepper, A.D. & Van Blitterswijk, W.J. Ceramide mass analysis by normal-phase high-performance liquid chromatography. Methods Enzymol. 312, 16–22 (2000).
Sullards, M.C. & Merrill, A.H., Jr. Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci STKE 2001, PL1 (2001).
Berdyshev, E.V., Gorshkova, I.A., Garcia, J.G.N., Natarajan, V. & Hubbard, W.C. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 339, 129–136 (2005).
Acknowledgements
The authors acknowledge the technical assistance of L. Natarajan, S.I. Ramirez, J. Skirball (cell culture and caspase-3 activity assay) and D. Rau (HPLC). We thank D. Griffin who provided the acid sphingomyelinase–deficient human fibroblasts. We thank C. Cool for providing additional normal human lung samples, and A.M.K. Choi and J.G.N. Garcia for critically reading our manuscript. Funding for these studies is from the US National Institutes of Health grant K08 HL04396; American Thoracic Society/Alpha One Foundation Research Grant; and American Lung Association Research Grant (to I.P.); US National Institutes of Health grants RO1 HL71152 (to V.N.), 1S10 RR16798 (to W.C.H.) and RO1HL66554 (to R.M.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic representation of ceramide as an amplifying mediator in pulmonary emphysema based on the findings in the VEGFR blockade model. (PDF 118 kb)
Supplementary Fig. 2
VEGFR blockade rapidly induces apoptosis in the mouse lung. (PDF 101 kb)
Supplementary Fig. 3
De novo ceramide synthesis is necessary for the development of emphysema, preceding caspase activation. (PDF 122 kb)
Supplementary Fig. 4
Ceramide augmentation causes cell apoptosis and oxydative stress in the mouse lung. (PDF 216 kb)
Supplementary Fig. 5
ASMase regulates paracellular amplification of ceramide synthesis. (PDF 188 kb)
Supplementary Table 1
Clinical characteristics of patients who provided lung biopsy samples for ceramide analysis. (PDF 38 kb)
Rights and permissions
About this article
Cite this article
Petrache, I., Natarajan, V., Zhen, L. et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11, 491–498 (2005). https://doi.org/10.1038/nm1238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1238
This article is cited by
-
Lipidomic alterations in human saliva from cystic fibrosis patients
Scientific Reports (2023)
-
Role of Lysocardiolipin Acyltransferase in Cigarette Smoke-Induced Lung Epithelial Cell Mitochondrial ROS, Mitochondrial Dynamics, and Apoptosis
Cell Biochemistry and Biophysics (2022)
-
Characterization of the COPD alveolar niche using single-cell RNA sequencing
Nature Communications (2022)
-
Role of ASM/Cer/TXNIP signaling module in the NLRP3 inflammasome activation
Lipids in Health and Disease (2021)
-
Role and mechanisms of autophagy in lung metabolism and repair
Cellular and Molecular Life Sciences (2021)