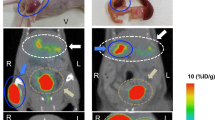
Abstract
Islet transplantation is an attractive approach for treating type-1 diabetes, but there is a massive loss of transplanted islets. It is currently only possible to estimate islet mass indirectly, through measurement of circulating C-peptide and insulin levels. This type of estimation, however, is not sufficiently sensitive or reproducible for follow-up of individuals who have undergone islet transplantation. Here we show that islet graft survival could be assessed for 1 month in diabetic NOD mice using 9-(4-[18F]-fluoro-3-hydroxymethylbutyl)guanine ([18F]FHBG)–positron emission tomography (PET) technology, the PET signal reflecting insulin secretory capacity of transplanted islets. Expression of the gene encoding viral interleukin-10 (vIL-10), was measurable in real time with PET scanning. Additionally, we addressed the clinical potential of this approach by visualizing transplanted islets in the liver, the preferred clinical transplantation site. We conclude that quantitative in vivo PET imaging is a valid method for facilitating the development of protocols for prolonging islet survival, with the potential for tracking human transplants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shapiro, A.M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
Hathout, E., Lakey, J. & Shapiro, J. Islet transplant: an option for childhood diabetes. Arch. Dis. Child. 88, 591–594 (2003).
Ryan, E.A. et al. Five-year follow-up after clinical islet transplantation. Diabetes 54, 2060–2069 (2005).
Shapiro, A.M. et al. Novel approaches toward early diagnosis of islet allograft rejection. Transplantation 71, 1709–1718 (2001).
Yu, Y. et al. Quantification of target gene expression by imaging reporter gene expression in living animals. Nat. Med. 6, 933–937 (2000).
Gambhir, S.S. et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc. Natl. Acad. Sci. USA 97, 2785–2790 (2000).
Massoud, T. & Gambhir, S.S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580 (2003).
Herschman, H.R. Micro-PET imaging and small animal models of disease. Curr. Opin. Immunol. 15, 378–384 (2003).
Chwalinski, S. & Potten, C.S. Crypt base columnar cells in ileum of BDF1 male mice—their numbers and some features of their proliferation. Am. J. Anat. 186, 397–406 (1989).
Yang, Z. et al. Suppression of autoimmune diabetes by viral IL-10 gene transfer. J. Immunol. 168, 6479–6485 (2002).
Carter, J.D. et al. Viral IL-10-mediated immune regulation in pancreatic islet transplantation. Mol. Ther. 12, 360–368 (2005).
Stock, P.G. & Bluestone, J.A. Beta-cell replacement for type 1 diabetes. Annu. Rev. Med. 55, 133–156 (2004).
Robertson, R.P. Islet transplantation as a treatment for diabetes – a work in progress. N. Engl. J. Med. 350, 694–705 (2004).
Jirak, D. et al. MRI of transplanted pancreatic islets. Magn. Reson. Med. 52, 1228–1233 (2004).
Evgenov, N.V., Medarova, Z., Dai, G., Bonner-Weir, S. & Moore, A. In vivo imaging of islet transplantation. Nat. Med. 12, 144–148 (2006).
Oca-Cossio, J. et al. Magnetically labeled insulin-secreting cells. Biochem. Biophys. Res. Commun. 319, 569–575 (2004).
Paty, B.W., Bonner-Weir, S., Laughlin, M.R., McEwan, A.J. & Shapiro, A.M.J. Toward development to imaging modalities from islets after transplantation: insights from the National Institutes of Health workshop on beta cell imaging. Transplantation 77, 1133–1137 (2004).
Lu, Y. et al. Bioluminescent monitoring of islet graft survival after transplantation. Mol. Ther. 9, 428–435 (2004).
Fowler, M. et al. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation 79, 768–776 (2005).
Salvalaggio, P.R. et al. Islet filtration: a simple and rapid new purification procedure that avoids ficoll and improves islet mass and function. Transplantation 74, 877–879 (2002).
Becker, T.C. et al. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell. Biol. 43, 161–189 (1994).
Ponde, D.E., Dence, C.S., Schuster, D.P. & Welch, M.J. Rapid and reproducible radiosynthesis of [18F]FHBG. Nucl. Med. Biol. 31, 133–138 (2004).
Qi, J., Leahy, R.M., Hsu, C., Farquhar, T.H. & Cherry, S.R. Fully 3D Bayesian image reconstruction for the ECAT EXACT HR+. IEEE Trans. Nucl. Sci. 45, 1096–1103 (1998).
Chatziioannou, A. et al. Comparison of 3-D maximum a posteriori and filtered back projection algorithms for high-resolution animal imaging with microPET. IEEE Trans. Med. Imaging 19, 507–512 (2000).
Acknowledgements
We would like to thank V. Sossi for assistance with data reconstruction, M.J. Adam for advice on [18F]FHBG radiosynthesis, S. McCormick and M. Speck for technical assistance, and M.E. Black (Washington State University) for antibody to HSV1-sr39TK. These studies were supported by funding to C.H.S.M. from the Canadian Institutes of Health Research (#590007) and the Canadian Foundation for Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Imunohistochemical staining of the left and right kidneys. (PDF 46 kb)
Supplementary Fig. 2
Imunohistochemical staining of islet transplanted kidneys. (PDF 92 kb)
Supplementary Fig. 3
The presence of DNA encoding vIL-10 in rAD-vIL10-ITK treated islet transplanted kidneys and plasma vIL-10 levels. (PDF 37 kb)
Supplementary Fig. 4
Cell viability on treatment with FHBG. Islets isolated from male C57BL/6 mice were infected with 250 m.o.i. of rAD-TK. (PDF 17 kb)
Supplementary Video 1
1 h-time course PET imaging of islets transplanted under the kidney capsule of C57 BL/6 mouse. (MOV 742 kb)
Supplementary Video 2
1 h-time course PET imaging of islet transplanted into the liver of C57 BL/6 mouse. (MOV 814 kb)
Rights and permissions
About this article
Cite this article
Kim, SJ., Doudet, D., Studenov, A. et al. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med 12, 1423–1428 (2006). https://doi.org/10.1038/nm1458
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1458
This article is cited by
-
B-Glycine as a marker for β cell imaging and β cell mass evaluation
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Tri-modal In vivo Imaging of Pancreatic Islets Transplanted Subcutaneously in Mice
Molecular Imaging and Biology (2018)
-
PET probes for imaging pancreatic islet cells
Clinical and Translational Imaging (2017)
-
Herpesviral capture of immunomodulatory host genes
Virus Genes (2017)
-
Imaging the islet graft by positron emission tomography
European Journal of Nuclear Medicine and Molecular Imaging (2012)