Abstract
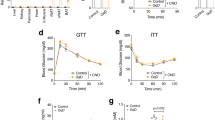
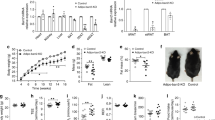
Thiazolidinediones (TZDs) are effective therapies for type 2 diabetes, which has reached epidemic proportions in industrialized societies. TZD treatment reduces circulating free fatty acids (FFAs), which oppose insulin actions in skeletal muscle and other insulin target tissues. Here we report that TZDs, acting as ligands for the nuclear receptor peroxisome proliferator-activated receptor (PPAR)-γ, markedly induce adipocyte glycerol kinase (GyK) gene expression. This is surprising, as standard textbooks indicate that adipocytes lack GyK and thereby avoid futile cycles of triglyceride breakdown and resynthesis from glycerol and FFAs. By inducing GyK, TZDs markedly stimulate glycerol incorporation into triglyceride and reduce FFA secretion from adipocytes. The 'futile' fuel cycle resulting from expression of GyK in adipocytes is thus a novel mechanism contributing to reduced FFA levels and perhaps insulin sensitization by antidiabetic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kopelman, P.G. Obesity as a medical problem. Nature 404, 635–643 (2000).
Flier, J.S. The adipocyte: Storage depot or node on the energy information superhighway? Cell 80, 15–18 (1995).
Trayhurn, P. & Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 60, 329–339 (2001).
Steppan, C.M. & Lazar, M.A. Resistin and obesity-associated insulin resistance. Trends Endocrinol. Metab. 13, 18–23 (2002).
Boden, G. Pathogenesis of type 2 diabetes. Insulin resistance. Endocrinol. Metab. Clin. North Am. 30, 801–815 (2001).
Roden, M. et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49, 701–707 (2000).
Griffin, M.E. et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48, 1270–1274 (1999).
Tontonoz, P., Hu, E., Graves, R.A., Budavari, A.I. & Spiegelman, B.M. mPPAR-γ2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 (1994).
Chawla, A., Schwarz, E.J., Dimaculangan, D.D. & Lazar, M.A. Peroxisome proliferator-activated receptor γ (PPAR-γ): Adipose predominant expression and induction early in adipocyte differentiation. Endocrinology 135, 798–800 (1994).
Barak, Y. et al. PPAR-γ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 (1999).
Rosen, E.D. et al. PPAR-γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 (1999).
Tontonoz, P., Hu, E., Devine, J., Beale, E.G. & Spiegelman, B.M. PPAR- γ 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 15, 351–357 (1995).
Mandrup, S. & Lane, M.D. Regulating adipogenesis. J. Biol. Chem. 272, 5367–5370 (1997).
Rosen, E.D. et al. C/EBPα induces adipogenesis through PPAR-γ: a unified pathway. Genes Dev. (in the press).
Yu, K. et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 270, 23975–23983 (1995).
Forman, B.M. et al. 15-deoxy, delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR-γ. Cell 83, 803–812 (1995).
Kliewer, S.A. et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 83, 813–819 (1995).
Lehmann, J.M. et al. An antidiabetic thiazolidinedione is a high affinity ligand for the nuclear peroxisome proliferator-activated receptor γ (PPAR-γ). J. Biol. Chem. 270, 12953–12956 (1995).
Mauvais-Jarvis, F., Andreelli, F., Hanaire-Broutin, H., Charbonnel, B. & Girard, J. Therapeutic perspectives for type 2 diabetes mellitus: molecular and clinical insights. Diabetes Metab. 27, 415–23 (2001).
Zhang, B. et al. Down-regulation of the expression of the obese gene by an antidiabetic thiazolidinedione in Zucker diabetic fatty rats and db/db mice. J. Biol. Chem. 271, 9455–9459 (1996).
Kallen, C.B. & Lazar, M.A. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 93, 5793–5796 (1996).
DeVos, P. et al. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. J. Clin. Invest. 98, 1004–1009 (1996).
Maeda, N. et al. PPAR-γ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099 (2001).
Steppan, C.M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Stumvoll, M. & Haring, H.U. Glitazones: Clinical effects and molecular mechanisms. Ann Med 34, 217–24 (2002).
Martin, G., Schoonjans, K., Lefebvre, A.M., Staels, B. & Auwerx, J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPAR-α and PPAR-γ activators. J. Biol. Chem. 272, 28210–28217 (1997).
Nagy, L., Tontonoz, P., Alvarez, J.G., Chen, H. & Evans, R.M. Oxidized LDL regulates macrophage gene expression through activation of PPAR-γ. Cell 93, 229–240 (1998).
Steinberg, D., Vaughan, M. & Margolis, S. Studies of triacylglyceride biosynthesis in homogenates of adipose tissue. J. Biol. Chem. 236, 1631–1637 (1961).
Jequier, E. & Tappy, L. Regulation of body weight in humans. Physiol. Rev. 79, 451–80 (1999).
Matthews, C.K. & van Holde, K.E. Metabolic coordination, metabolic control, and signal transduction. in Biochemistry (eds. Matthews, C.K. & vanHolde, K.E.) 819–859 (Benjamin/Cummings, Menlo Park, 1996).
Li, Y. & Lazar, M.A. Differential gene regulation by PPAR-γ agonist and constitutively active PPAR-γ. Mol. Endocrinol. 16, 1040–1048 (2002).
Camp, H.S., Chaudhry, A. & Leff, T. A novel potent antagonist of peroxisome proliferator-activated receptor γ blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinology 142, 3207–3213 (2001).
Kliewer, S.A. et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. USA 94, 4318–4323 (1997).
Ibrahimi, A. et al. Evidence for a common mechanism of action for fatty acids and thiazolidinedione antidiabetic agents on gene expression in preadipose cells. Mol. Pharmacol. 46, 1070–1076 (1994).
Mukherjee, R. et al. A selective peroxisome proliferator-activated receptor γ modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol. Endocrinol. 14, 1425–1433 (2000).
Schoonjans, K. et al. PPAR-α and PPAR-γ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 15, 5336–48 (1996).
Noel, R.J., Antinozzi, P.A., McGarry, J.D. & Newgard, C.B. Engineering of glycerol-stimulated insulin secretion in islet beta cells. Differential metabolic fates of glucose and glycerol provide insight into mechanisms of stimulus-secretion coupling. J. Biol. Chem. 272, 18621–7 (1997).
Kishida, K. et al. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator-activated receptor γ. J. Biol. Chem. 276, 48572–48579 (2001).
Krusynska, Y.T. Normal metabolism: the physiology of fuel homeostasis. in Textbook of Diabetes (eds. Pickup, J. & Williams, G.) 11.1–11.37 (Blackwell Science, Oxford, 1997).
Felig, P. & Bergman, M. Physiology of fuel metabolism. in Endocrinology and Metabolism (eds. Felig, P., Baxter, J.D. & Frohman, L.A.) 1107–1156 (McGraw-Hill, New York, 1995).
Souza, S.C., Yamamoto, M.T., Franciosa, M.D., Lien, P. & Greenberg, A.S. BRL 49653 blocks the lipolytic actions of tumor necrosis factor-α: A potential new insulin-sensitizing mechanism for thiazolidinediones. Diabetes 47, 691–695 (1998).
Gaudet, D. et al. Glycerol as a correlate of impaired glucose tolerance: dissection of a complex system by use of a simple genetic trait. Am. J. Hum. Genet. 66, 1558–1568 (2000).
Huq, A.H., Lovell, R.S., Ou, C.N., Beaudet, A.L. & Craigen, W.J. X-linked glycerol kinase deficiency in the mouse leads to growth retardation, altered fat metabolism, autonomous glucocorticoid secretion and neonatal death. Hum. Mol. Genet. 6, 1803–1809 (1997).
Kaplan, M.L. & Leveille, G.A. Development of lipogenesis and insulin sensitivity in tissues of the ob/ob mouse. Am. J. Physiol. 240, E101–107 (1981).
Air, E.L. et al. Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nature Med. 8, 179–183 (2002).
Ruan, H. & Pownall, H.J. Overexpression of 1-acyl-glycerol-3-phosphate acyltransferase-α enhances lipid storage in cellular models of adipose tissue and skeletal muscle. Diabetes 50, 233–240 (2001).
Acknowledgements
We thank H. Camp and D. Owens for the gift of PD068235; T. Willson for GW7845; W. Yin for help with the GyK assay; Y. Qi and R. Ahima for assistance with animal experiments; and M. Birnbaum for helpful discussions and critical reading of the manuscript. This work was supported by NIH grant DK49780 (to M.A.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.A.L received unrestricted research support from GlaxoSmithKline.
Rights and permissions
About this article
Cite this article
Guan, HP., Li, Y., Jensen, M. et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med 8, 1122–1128 (2002). https://doi.org/10.1038/nm780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm780
This article is cited by
-
Hyperphagia of female UCP1-deficient mice blunts anti-obesity effects of FGF21
Scientific Reports (2023)
-
Lipid cycling isn’t all futile
Nature Metabolism (2023)
-
Remodeling of Adipose Tissues by Fatty Acids: Mechanistic Update on Browning and Thermogenesis by n-3 Polyunsaturated Fatty Acids
Pharmaceutical Research (2023)
-
Resident and migratory adipose immune cells control systemic metabolism and thermogenesis
Cellular & Molecular Immunology (2022)
-
ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity
Reviews in Endocrine and Metabolic Disorders (2022)