Abstract
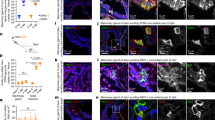
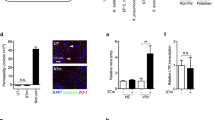
Epstein-Barr virus (EBV) initially enters the body through the oropharyngeal mucosa and subsequently infects B lymphocytes through their CD21 (CR2) complement receptor. Mechanisms of EBV entry into and release from epithelial cells are poorly understood. To study EBV infection in mucosal oropharyngeal epithelial cells, we established human polarized tongue and pharyngeal epithelial cells in culture. We show that EBV enters these cells through three CD21-independent pathways: (i) by direct cell-to-cell contact of apical cell membranes with EBV-infected lymphocytes; (ii) by entry of cell-free virions through basolateral membranes, mediated in part through an interaction between b1 or α5β1 integrins and the EBV BMRF-2 protein; and (iii) after initial infection, by virus spread directly across lateral membranes to adjacent epithelial cells. Release of progeny virions from polarized cells occurs from both their apical and basolateral membranes. These data indicate that multiple approaches to prevention of epithelial infection with EBV will be necessary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rickinson, A.B. & Kieff, E. Epstein-Barr virus. In Fields Virology (eds. Fields, B.N., Knipe, D.M. & Howley, P.M.) 2575–2627 (Lippincott-Williams & Wilkins, Philadelphia, 2001).
Tanner, J., Weis, J., Fearon, D., Whang, Y. & Kieff, E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50, 203–213 (1987).
Fingeroth, J.D. et al. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. USA 81, 4510–4514 (1984).
Molesworth, S.J., Lake, C.M., Borza, C.M., Turk, S.M. & Hutt-Fletcher, L.M. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J. Virol. 74, 6324–6332 (2000).
Wang, X. & Hutt-Fletcher, L.M. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72, 158–163 (1998).
Wang, X., Kenyon, W.J., Li, Q., Mullberg, J. & Hutt-Fletcher, L.M. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72, 5552–5558 (1998).
Li, Q. et al. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71, 4657–4662 (1997).
Haan, K.M., Kwok, W.W., Longnecker, R. & Speck, P. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. J. Virol. 74, 2451–2454 (2000).
Borza, C.M. & Hutt-Fletcher, L.M. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8, 594–599 (2002).
Greenspan, J.S. et al. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 313, 1564–1571 (1985).
Sixbey, J.W., Nedrud, J.G., Raab-Traub, N., Hanes, R.A. & Pagano, J.S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310, 1225–1230 (1984).
Lemon, S.M., Hutt, L.M., Shaw, J.E., Li, J.L. & Pagano, J.S. Replication of EBV in epithelial cells during infectious mononucleosis. Nature 268, 268–270 (1977).
Wolf, H., Haus, M. & Wilmes, E. Persistence of Epstein-Barr virus in the parotid gland. J. Virol. 51, 795–798 (1984).
Hurley, E.A., Klaman, L.D., Agger, S., Lawrence, J.B. & Thorley-Lawson, D.A. The prototypical Epstein-Barr virus-transformed lymphoblastoid cell line IB4 is an unusual variant containing integrated but no episomal viral DNA. J. Virol. 65, 3958–3963 (1991).
Zhang, J.R. et al. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102, 827–837 (2000).
Simons, K. & Fuller, S.D. Cell surface polarity in epithelia. Annu. Rev. Cell Biol. 1, 243–288 (1985).
Imai, S., Nishikawa, J. & Takada, K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72, 4371–4378 (1998).
Chang, Y. et al. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J. Virol. 73, 8857–8866 (1999).
Modrow, S., Höflacher, B. & Wolf, I.I. Identification of a protein encoded in the EB-viral open reading frame BMRF2. Arch. Virol. 127, 379–386 (1992).
Gavrilovskaya, I.N., Brown, E.J., Ginsberg, M.H. & Mackow, E.R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 73, 3951–3959 (1999).
Nemerow, G.R. Cell receptors involved in adenovirus entry. Virology 274, 1–4 (2000).
Jackson, T. et al. Arginine-glycine-aspartic acid-specific binding by foot-and-mouth disease viruses to the purified integrin αvβ3 in vitro. J. Virol. 71, 8357–8361 (1997).
Jackson, T., Sheppard, D., Denyer, M., Blakemore, W. & King, A.M. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74, 4949–4956 (2000).
Akula, S.M., Pramod, N.P., Wang, F.Z. & Chandran, B. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108, 407–419 (2002).
Plow, E.F., Haas, T.A., Zhang, L., Loftus, J. & Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 (2000).
Yoshiyama, H., Imai, S., Shimizu, N. & Takada, K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J. Virol. 71, 5688–5691 (1997).
Janz, A. et al. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 74, 10142–10152 (2000).
Chang, Y. et al. Detection of transcripts initiated from two viral promoters (Cp and Wp) in Epstein-Barr virus-infected nasopharyngeal carcinoma cells and biopsies. Lab. Invest. 78, 715–726 (1998).
Palefsky, J.M. et al. Epstein-Barr virus BMRF-2 and BDLF-3 expression in hairy leukoplakia. Oral Dis. 3 suppl. 1, S171–176 (1997).
Peñaranda, M.E. et al. Expression of Epstein-Barr virus BMRF-2 and BDLF-3 genes in hairy leukoplakia [published erratum appears in J. Gen. Virol. 79, 1321 (1998)]. J. Gen. Virol. 78, 3361–3370 (1997).
Lagenaur, L.A. & Palefsky, J.M. Regulation of Epstein-Barr virus promoters in oral epithelial cells and lymphocytes. J. Virol. 73, 6566–6572 (1999).
Akiyama, S.K. & Yamada, K.M. Biosynthesis and acquisition of biological activity of the fibronectin receptor. J. Biol. Chem. 262, 17536–17542 (1987).
Kim, L.T. et al. Altered glycosylation and cell surface expression of β1 integrin receptors during keratinocyte activation. J. Cell Sci. 103, 743–753 (1992).
Friedl, P., Zanker, K.S. & Brocker, E.B. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc. Res. Tech. 43, 369–378 (1998).
Friedl, P., Brocker, E.B. & Zanker, K.S. Integrins, cell matrix interactions and cell migration strategies: fundamental differences in leukocytes and tumor cells. Cell Adhes. Commun. 6, 225–236 (1998).
Iwata, S., Ohashi, Y., Kamiguchi, K. & Morimoto, C. β1-integrin-mediated cell signaling in T lymphocytes. J. Dermatol. Sci. 23, 75–86 (2000).
Giancotti, F.G. & Ruoslahti, E. Integrin signaling. Science 285, 1028–1032 (1999).
Tugizov, S., Maidji, E. & Pereira, L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J. Gen. Virol. 77, 61–74 (1996).
Dingwell, K.S. et al. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68, 834–845 (1994).
Johnson, D.C. & Huber, M.T. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76, 1–8 (2002).
Tugizov, S., Maidji, E., Xiao, J., Zheng, Z. & Pereira, L. Human cytomegalovirus glycoprotein B contains autonomous determinants for vectorial targeting to apical membranes of polarized epithelial cells. J. Virol. 72, 7374–7386 (1998).
Caplan, M.J., Anderson, H.C., Pallade, G.E. & Jamieson, J.D. Intracellular sorting and polarized cell surface delivery of (Na+, K+) ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell 46, 623–631 (1986).
Lembo, D., Angeretti, A., Gariglio, M. & Landolfo, S. Murine cytomegalovirus induces expression and enzyme activity of cellular dihydrofolate reductase in quiescent cells. J. Gen. Virol. 79, 2803–2807 (1998).
Hall, D.E. et al. The α1/β1 and α6/β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J. Cell Biol. 110, 2175–2184 (1990).
Werb, Z., Tremble, P.M., Behrendtsen, O., Crowley, E. & Damsky, C.H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J. Cell Biol. 109, 877–889 (1989).
Hanzel, D., Nabi, I.R., Surzolo, C., Powell, S.K. & Rodriguez-Boulan, E. New techniques lead to advances in epithelial cell polarity. Sem. Cell Biol. 2, 341–353 (1991).
Acknowledgements
We thank E. Lennette for sera from nasopharyngeal carcinoma patients, V. Petersen for electron microscopy and P. Dazin for cell sorting assays. This project was supported by US National Institutes of Health grant P01 DE07946 and funds provided by the Division of Research Resources 5 M01-RR-00079, US Public Health Service.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tugizov, S., Berline, J. & Palefsky, J. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med 9, 307–314 (2003). https://doi.org/10.1038/nm830
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm830
This article is cited by
-
SuPAR mediates viral response proteinuria by rapidly changing podocyte function
Nature Communications (2023)
-
A high-throughput neutralizing assay for antibodies and sera evaluation against Epstein-Barr virus
Virology Journal (2022)
-
Vaccination against the Epstein–Barr virus
Cellular and Molecular Life Sciences (2020)
-
Latency and lytic replication in Epstein–Barr virus-associated oncogenesis
Nature Reviews Microbiology (2019)
-
Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study
Virchows Archiv (2019)