Abstract
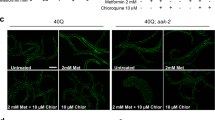
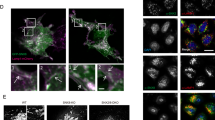
Inhibition of polyglutamine-induced protein aggregation could provide treatment options for polyglutamine diseases such as Huntington disease. Here we showed through in vitro screening studies that various disaccharides can inhibit polyglutamine-mediated protein aggregation. We also found that various disaccharides reduced polyglutamine aggregates and increased survival in a cellular model of Huntington disease. Oral administration of trehalose, the most effective of these disaccharides, decreased polyglutamine aggregates in cerebrum and liver, improved motor dysfunction and extended lifespan in a transgenic mouse model of Huntington disease. We suggest that these beneficial effects are the result of trehalose binding to expanded polyglutamines and stabilizing the partially unfolded polyglutamine-containing protein. Lack of toxicity and high solubility, coupled with efficacy upon oral administration, make trehalose promising as a therapeutic drug or lead compound for the treatment of polyglutamine diseases. The saccharide-polyglutamine interaction identified here thus provides a new therapeutic strategy for polyglutamine diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zoghbi, H.Y. & Orr, H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Scherzinger, E. et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90, 549–558 (1997).
Davies, S.W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
Warrick, J.M. et al. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93, 939–949 (1998).
Lunkes, A. & Mandel, J.L. A cellular model that recapitulates major pathogenic steps of Huntington's disease. Hum. Mol. Genet. 7, 1355–1361 (1998).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Michalik, A. & Van Broeckhoven, C. Pathogenesis of polyglutamine disorders: aggregation revisited. Hum. Mol. Genet. 12, R173–R186 (2003).
Mccampbell, A. & Fischbeck, K.H. Polyglutamine and CBP: fatal attraction? Nat. Med. 7, 528–530 (2001).
Ross, C.A., Poirier, M.A., Wanker, E.E. & Amzel, M. Polyglutamine fibrillogenesis: the pathway unfolds. Proc. Natl. Acad. Sci. USA 100, 1–3 (2003).
Bates, G. Huntingtin aggregation and toxicity in Huntington's disease. Lancet 361, 1642–1644 (2003).
Ona, V.O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).
Cummings, C.J. et al. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat. Genet. 19, 148–154 (1998).
Jana, N.R., Tanaka, M., Wang, G.H. & Nukina, N. Polyglutamine length–dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum. Mol. Genet. 9, 2009–2018 (2000).
Shimohata, T. et al. Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat. Genet. 26, 29–36 (2000).
Perez, M.K. et al. Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J. Cell. Biol. 143, 1457–1470 (1998).
Chen, M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6, 797–801 (2000).
Ferrante, R.J. et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 20, 4389–4397 (2000).
Hockly, E. et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc. Natl. Acad. Sci. USA 100, 2041–2046 (2003).
Sanchez, I., Mahlke, C. & Yuan, J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421, 373–379 (2003).
Hughes, R.E. & Olson, J.M. Therapeutic opportunities in polyglutamine disease. Nat. Med. 7, 419–423 (2001).
Heiser, V. et al. Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implication for Huntington's disease therapy. Proc. Natl. Acad. Sci. USA 97, 6739–6744 (2000).
Heiser, V. et al. Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington's disease by using an automated filter retardation assay. Proc. Natl. Acad. Sci. USA 99, 16400–16406 (2002).
Tanaka, M., Morishima, I., Akagi, T., Hashikawa, T. & Nukina, N. Intra- and intermolecular β-pleated sheet formation in glutamine-repeat inserted myoglobin as a model for polyglutamine diseases. J. Biol. Chem. 276, 45470–45475 (2001).
Tanaka, M. et al. The effects of aggregation-inducing motifs on amyloid formation of model proteins related to neurodegenerative diseases. Biochemistry 41, 10277–10286 (2002).
Nagai, Y. et al. Inhibition of polyglutamine protein aggregation and cell death by novel peptides identified by phage display screening. J. Biol. Chem. 275, 10437–10442 (2000).
Luo, Y. & Baldwin, R.L. Trifluoroethanol stabilizes the pH 4 folding intermediate of sperm whale apomyoglobin. J. Mol. Biol. 279, 49–57 (1998).
Wang, G.H. et al. Caspase activation during apoptotic cell death induced by expanded polyglutamine in N2a cells. Neuroreport 10, 2435–2438 (1999).
Guo, N., Puhlev, I., Brown, D.R., Mansbridge, J. & Levine, F. Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol. 18, 168–171 (2000).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996).
Sathasivam, K. et al. Formation of polyglutamine inclusions in non-CNS tissue. Hum. Mol. Genet. 8, 813–822 (1999).
Poirier, M.A. et al. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J. Biol. Chem. 277, 41032–41037 (2002).
Azzouz, M. et al. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum. Mol. Genet. 9, 803–811 (2000).
Hurlbert, M.S. et al. Mice transgenic for an expanded CAG repeat in the Huntington's disease gene develop diabetes. Diabetes 48, 649–651 (1999).
Farrer, A. Diabetes mellitus in Huntington's disease. Clin. Genet. 27, 62–67 (1985).
Singer, M.A. & Lindquist, S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1, 639–648 (1998).
Kazantsev, A. et al. A bivalent Huntingtin binding peptide suppresses polyglutamine aggregation and pathogenesis in Drosophila. Nat. Genet. 30, 367–376 (2002).
Yang, W., Dunlap, J.R., Andrews, R.B. & Wetzel, R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum. Mol. Genet. 11, 2905–2917 (2002).
Wellington, C.L. et al. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 273, 9158–9167 (1998).
Kandror, O., DeLeon, A. & Goldberg, A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperature. Proc. Natl. Acad. Sci. USA 99, 9727–9732 (2002).
Acknowledgements
We thank Y. Yamada (RIKEN Brain Science Institute) for maintenance of mice, N. Yoshiki and Y. Obata (RIKEN Tsukuba institute) for instruction on ovarian transplantation of R6/2 transgenic mice, and S. Sligar (University of Illinois) for allowing us to use the wild-type myoglobin expression plasmid. This study was partly supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (M.T. and N.N.) and of Health, Labour and Welfare (N.N), Japan, and by RIKEN President's Special Research Grant (M.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tanaka, M., Machida, Y., Niu, S. et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10, 148–154 (2004). https://doi.org/10.1038/nm985
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm985
This article is cited by
-
A clinical study and future prospects for bioactive compounds and semi-synthetic molecules in the therapies for Huntington's disease
Molecular Neurobiology (2024)
-
Highly concentrated trehalose induces prohealing senescence-like state in fibroblasts via CDKN1A/p21
Communications Biology (2023)
-
A neuroprotective dose of trehalose is harmless to metabolic organs: comprehensive histopathological analysis of liver, pancreas, and kidney
DARU Journal of Pharmaceutical Sciences (2023)
-
Bicalutamide and Trehalose Ameliorate Spinal and Bulbar Muscular Atrophy Pathology in Mice
Neurotherapeutics (2023)
-
Intake of l-serine before bedtime prevents the delay of the circadian phase in real life
Journal of Physiological Anthropology (2022)