Abstract
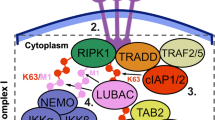
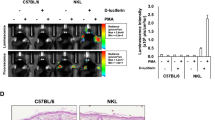
The transcription factor NF-κB is a key regulator of cellular activation, proliferation and apoptosis. Defects in the NF-κB pathway contribute to a broad array of malignant, neurodegenerative and chronic inflammatory diseases. IKK-dependent IκBα degradation by the 26S proteasome is a critical NF-κB regulatory control point, which is emerging as an important target for drug development. To directly monitor regulation of IKK activation in intact organisms, we engineered an IκBα–firefly luciferase (IκBα-FLuc) fusion reporter. In cultured cells and living animals, the reporter provided a continuous, noninvasive readout of the kinetics of ligand-induced IKK activation and the pharmacodynamics of selective inhibitors of both IKK and the 26S proteasome. This IκBα-FLuc reporter now permits continuous readout of IKK activation in vivo, facilitates development and validation of target-specific therapeutics, and complements conventional NF-κB transcriptional reporters for more complete temporal and regional investigations of the NF-κB signaling pathway in health and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Karin, M., Cao, Y., Greten, F.R. & Li, Z.W. NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2, 301–310 (2002).
Li, Q. & Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Aggarwal, B.B. Nuclear factor κB: the enemy within. Cancer Cell 6, 203–208 (2004).
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell 109 (Suppl.), S81–S96 (2002).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 (2000).
Hayden, M.S. & Ghosh, S. Signaling to NF-κB. Genes Dev. 18, 2195–2224 (2004).
Greten, F.R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Lawrence, T., Bebien, M., Liu, G.Y., Nizet, V. & Karin, M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434, 1138–1143 (2005).
Hay, R.T. Modifying NEMO. Nat. Cell Biol. 6, 89–91 (2004).
Karin, M., Yamamoto, Y. & Wang, Q.M. The IKK NF-κB system: a treasure trove for drug development. Nat. Rev. Drug Discov. 3, 17–26 (2004).
Luker, K.E. et al. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc. Natl. Acad. Sci. USA 101, 12288–12293 (2004).
Jacobs, M.D. & Harrison, S.C. Structure of an IκBα/NF-κB complex. Cell 95, 749–758 (1998).
Barroga, C.F., Stevenson, J.K., Schwarz, E.M. & Verma, I.M. Constitutive phosphorylation of IκBα by casein kinase II. Proc. Natl. Acad. Sci. USA 92, 7637–7641 (1995).
Place, R.F., Haspeslagh, D., Hubbard, A.K. & Giardina, C. Cytokine-induced stabilization of newly synthesized IκB-α. Biochem. Biophys. Res. Commun. 283, 813–820 (2001).
Henkel, T. et al. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 365, 182–185 (1993).
Lin, Z.P. et al. Prevention of brefeldin A–induced resistance to teniposide by the proteasome inhibitor MG-132: involvement of NF-κB activation in drug resistance. Cancer Res. 58, 3059–3065 (1998).
Luker, G., Pica, C., Song, J., Luker, K. & Piwnica-Worms, D. Imaging 26S proteasome activity and inhibition in living mice. Nat. Med. 9, 969–973 (2003).
Tan, C. & Waldmann, T.A. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 62, 1083–1086 (2002).
An, J., Fisher, M. & Rettig, M.B. VHL expression in renal cell carcinoma sensitizes to bortezomib (PS-341) through an NF-κB–dependent mechanism. Oncogene 24, 1563–1570 (2005).
May, M.J. et al. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289, 1550–1554 (2000).
Pierce, J.W. et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103 (1997).
Hideshima, T. et al. NF-κB as a therapeutic target in multiple myeloma. J. Biol. Chem. 277, 16639–16647 (2002).
Lin, Y., Yao, S., Veach, R., Torgenson, T. & Hawiger, J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270, 14255–14258 (1995).
Streetz, K.L. et al. Lack of gp130 expression in hepatocytes promotes liver injury. Gastroenterology 125, 532–543 (2003).
Liu, F., Song, Y. & Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 (1999).
Bross, P.F. et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Cancer Res. 10, 3954–3964 (2004).
Ravi, R. & Bedi, A. NF-κB in cancer—a friend turned foe. Drug Resist. Updat. 7, 53–67 (2004).
Castro, A.C. et al. Novel IKK inhibitors: beta-carbolines. Bioorg. Med. Chem. Lett. 13, 2419–2422 (2003).
Imbert, V. et al. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell 86, 787–798 (1996).
Carlsen, H., Moskaug, J.O., Fromm, S.H. & Blomhoff, R. In vivo imaging of NF-κB activity. J. Immunol. 168, 1441–1446 (2002).
Magness, S.T. et al. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-κB activation using a novel gene-targeted enhanced GFP reporter gene mouse. J. Immunol. 173, 1561–1570 (2004).
Acknowledgements
The authors thank S. Gammon for insightful discussions and help with statistical analyses. Funded by National Institutes of Health grant P50 CA94056.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Gross, S., Piwnica-Worms, D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods 2, 607–614 (2005). https://doi.org/10.1038/nmeth779
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth779
This article is cited by
-
TLR5 agonists enhance anti-tumor immunity and overcome resistance to immune checkpoint therapy
Communications Biology (2023)
-
A novel 2-iminobenzimidazole compound, XYA1353, displays in vitro and in vivo anti-myeloma activity via targeting NF-κB signaling
Molecular and Cellular Biochemistry (2023)
-
KappaBle fluorescent reporter mice enable low-background single-cell detection of NF-κB transcriptional activity in vivo
Mucosal Immunology (2022)
-
Glucuronidation of d-Luciferin In Vitro: Isoform Selectivity and Kinetics Characterization
European Journal of Drug Metabolism and Pharmacokinetics (2019)
-
High-resolution clonal mapping of multi-organ metastasis in triple negative breast cancer
Nature Communications (2018)