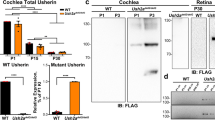
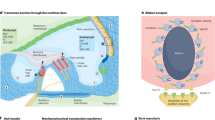
Abstract
The identification of mutations underlying monogenic, early-onset forms of deafness in humans has provided unprecedented insight into the molecular mechanisms of hearing in the peripheral auditory system. The molecules involved in the development and function of the cochlea eluded characterization until recently owing to the scarcity of the principal cell types present. The genetic approach has circumvented this problem and succeeded in identifying proteins and deciphering some of the molecular complexes that operate in these cells. In combination with mouse models, the genetic approach is now revealing some of the principles underlying the development and physiology of the cochlea. Focusing on the hair bundle, the mechanosensory device of the sensory hair cell, we highlight recent advances in understanding the way in which the hair bundle is formed, how it operates as a mechanotransducer and how it processes sound. In particular, we discuss how these findings confer a central role on the various hair-bundle links in these processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bregman, A.S. Auditory Scene Analysis: The Perceptual Organization of Sound (MIT Press, Cambridge, Massachusetts, USA, 1990).
Moore, B.C., Tyler, L.K. & Marslen-Wilson, W. (eds.). The Perception of Speech: From Sound to Meaning vol. 363 (Royal Society, London, 2008).
Ohm, G.S. Über die Definition des Tones, nebst daran geknüpfter Theorie der Sirene und ähnlicher tonbildender Vorrichtungen. Annalen der Physik und Chemie 59, 513–565 (1843).
von Helmholtz, H. Die Lehre von den Tonempfindungen als physiologische Grundlage fur die Theorie der Musik [On the Sensations of Tone as a Physiological Basis for the Theory of Music] (Vieweg, Braunschweig, 1862).
Gold, T. Hearing. II. The physical basis of the action of the cochlea. Proc. R. Soc. Lond. B 135, 492–498 (1948).
Petit, C. Genes responsible for human hereditary deafness: symphony of a thousand. Nat. Genet. 14, 385–391 (1996).
Frolenkov, G.I., Belyantseva, I.A., Friedman, T.B. & Griffith, A.J. Genetic insights into the morphogenesis of inner ear hair cells. Nat. Rev. Genet. 5, 489–498 (2004).
Friedman, L.M., Dror, A.A. & Avraham, K.B. Mouse models to study inner ear development and hereditary hearing loss. Int. J. Dev. Biol. 51, 609–631 (2007).
Brown, S.D., Hardisty-Hughes, R.E. & Mburu, P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat. Rev. Genet. 9, 277–290 (2008).
Richardson, G.P., Lukashkin, A.N. & Russell, I.J. The tectorial membrane: one slice of a complex cochlear sandwich. Curr. Opin. Otolaryngol. Head Neck Surg. 16, 458–464 (2008).
Pickles, J.O., Comis, S.D. & Osborne, M.P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103–112 (1984).
Furness, D.N. & Hackney, C.M. Cross-links between stereocilia in the guinea pig cochlea. Hear. Res. 18, 177–188 (1985).
Tsuprun, V. & Santi, P. Structure of outer hair cell stereocilia links in the chinchilla. J. Neurocytol. 27, 517–528 (1998).
Tsuprun, V. & Santi, P. Structure of outer hair cell stereocilia side and attachment links in the chinchilla cochlea. J. Histochem. Cytochem. 50, 493–502 (2002).
Tsuprun, V., Schachern, P.A., Cureoglu, S. & Paparella, M. Structure of the stereocilia side links and morphology of auditory hair bundle in relation to noise exposure in the chinchilla. J. Neurocytol. 32, 1117–1128 (2003).
Goodyear, R.J., Marcotti, W., Kros, C.J. & Richardson, G.P. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J. Comp. Neurol. 485, 75–85 (2005).
Hudspeth, A.J. Extracellular current flow and the site of transduction by vertebrate hair cells. J. Neurosci. 2, 1–10 (1982).
Jaramillo, F. & Hudspeth, A.J. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron 7, 409–420 (1991).
Lumpkin, E.A. & Hudspeth, A.J. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc. Natl. Acad. Sci. USA 92, 10297–10301 (1995).
Corey, D.P. & Hudspeth, A.J. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 3, 962–976 (1983).
Assad, J.A., Shepherd, G.M. & Corey, D.P. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994 (1991).
Howard, J. & Hudspeth, A.J. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc. Natl. Acad. Sci. USA 84, 3064–3068 (1987).
Crawford, A.C. & Fettiplace, R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J. Physiol. (Lond.) 364, 359–379 (1985).
Ricci, A.J., Crawford, A.C. & Fettiplace, R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J. Neurosci. 20, 7131–7142 (2000).
Martin, P., Bozovic, D., Choe, Y. & Hudspeth, A.J. Spontaneous oscillation by hair bundles of the bullfrog's sacculus. J. Neurosci. 23, 4533–4548 (2003).
Martin, P. & Hudspeth, A.J. Active hair-bundle movements can amplify a hair cell's response to oscillatory mechanical stimuli. Proc. Natl. Acad. Sci. USA 96, 14306–14311 (1999).
Martin, P. & Hudspeth, A.J. Compressive nonlinearity in the hair bundle's active response to mechanical stimulation. Proc. Natl. Acad. Sci. USA 98, 14386–14391 (2001).
Kennedy, H.J., Crawford, A.C. & Fettiplace, R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433, 880–883 (2005).
Eatock, R.A., Corey, D.P. & Hudspeth, A.J. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J. Neurosci. 7, 2821–2836 (1987).
Assad, J.A., Hacohen, N. & Corey, D.P. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc. Natl. Acad. Sci. USA 86, 2918–2922 (1989).
Crawford, A.C., Evans, M.G. & Fettiplace, R. Activation and adaptation of transducer currents in turtle hair cells. J. Physiol. (Lond.) 419, 405–434 (1989).
Crawford, A.C., Evans, M.G. & Fettiplace, R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J. Physiol. (Lond.) 434, 369–398 (1991).
Hudspeth, A.J. Making an effort to listen: mechanical amplification in the ear. Neuron 59, 530–545 (2008).
Cheung, E.L. & Corey, D.P. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys. J. 90, 124–139 (2006).
Beurg, M., Nam, J.H., Crawford, A. & Fettiplace, R. The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys. J. 94, 2639–2653 (2008).
Tilney, L.G., Tilney, M.S. & DeRosier, D.J. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 8, 257–274 (1992).
Cotanche, D.A. & Corwin, J.T. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear. Res. 52, 379–402 (1991).
Kaltenbach, J.A., Falzarano, P.R. & Simpson, T.H. Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J. Comp. Neurol. 350, 187–198 (1994).
Boeda, B. et al. Myosin VIIa, harmonin, and cadherin 23, three Usher I gene products, cooperate to shape the sensory hair cell bundle. EMBO J. 21, 6689–6699 (2002).
Michalski, N. et al. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J. Neurosci. 27, 6478–6488 (2007).
Manor, U. & Kachar, B. Dynamic length regulation of sensory stereocilia. Semin. Cell Dev. Biol. 19, 502–510 (2008).
Curtin, J.A. et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 13, 1129–1133 (2003).
Montcouquiol, M. et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173–177 (2003).
Lu, X. et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430, 93–98 (2004).
Wang, Y., Guo, N. & Nathans, J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147–2156 (2006).
Qian, D. et al. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121–133 (2007).
Etheridge, S.L. et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 4, e1000259 (2008).
Yamamoto, S. et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 15, 23–36 (2008).
Wang, J. et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 37, 980–985 (2005).
Montcouquiol, M. et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265–5275 (2006).
Ross, A.J. et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135–1140 (2005).
Jones, C. et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69–77 (2008).
Habas, R., Dawid, I.B. & He, X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295–309 (2003).
Furness, D.N., Richardson, G.P. & Russell, I.J. Stereociliary bundle morphology in organotypic cultures of the mouse cochlea. Hear. Res. 38, 95–109 (1989).
Pickles, J.O., von Perger, M., Rouse, G.W. & Brix, J. The development of links between stereocilia in hair cells of the chick basilar papilla. Hear. Res. 54, 153–163 (1991).
Waguespack, J., Salles, F.T., Kachar, B. & Ricci, A.J. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J. Neurosci. 27, 13890–13902 (2007).
Goodyear, R.J. & Richardson, G.P. A novel antigen sensitive to calcium chelation that is associated with the tip links and kinocilial links of sensory hair bundles. J. Neurosci. 23, 4878–4887 (2003).
Siemens, J. et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950–955 (2004).
Sollner, C. et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428, 955–959 (2004).
Lagziel, A. et al. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev. Biol. 280, 295–306 (2005).
Michel, V. et al. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev. Biol. 280, 281–294 (2005).
Ahmed, Z.M. et al. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J. Neurosci. 26, 7022–7034 (2006).
Kazmierczak, P. et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91 (2007).
Adato, A. et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 14, 3921–3932 (2005).
McGee, J. et al. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J. Neurosci. 26, 6543–6553 (2006).
Goodyear, R.J. et al. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J. Neurosci. 23, 9208–9219 (2003).
Verpy, E. et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature 456, 255–258 (2008).
Lefevre, G. et al. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development 135, 1427–1437 (2008).
Leibovici, M., Safieddine, S. & Petit, C. Mouse models of human hereditary deafness. Curr. Top. Dev. Biol. 84, 385–429 (2008).
Furness, D.N., Mahendrasingam, S., Ohashi, M., Fettiplace, R. & Hackney, C.M. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J. Neurosci. 28, 6342–6353 (2008).
Henn, A. & De La Cruz, E.M. Vertebrate myosin VIIb is a high duty ratio motor adapted for generating and maintaining tension. J. Biol. Chem. 280, 39665–39676 (2005).
El-Amraoui, A., Bahloul, A. & Petit, C. Myosin VII. in Myosins: A Superfamily of Molecular Motors vol. 7 (Coluccio, L.M., ed.) 353–373 (Springer, New York, 2008).
Wang, A. et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science 280, 1447–1451 (1998).
Mburu, P. et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet. 34, 421–428 (2003).
Donaudy, F. et al. Espin gene (ESPN) mutations associated with autosomal dominant hearing loss cause defects in microvillar elongation or organisation. J. Med. Genet. 43, 157–161 (2006).
Naz, S. et al. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J. Med. Genet. 41, 591–595 (2004).
Prosser, H.M., Rzadzinska, A.K., Steel, K.P. & Bradley, A. Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol. Cell. Biol. 28, 1702–1712 (2008).
Nayak, G.D., Ratnayaka, H.S., Goodyear, R.J. & Richardson, G.P. Development of the hair bundle and mechanotransduction. Int. J. Dev. Biol. 51, 597–608 (2007).
Oganesian, A. et al. Protein tyrosine phosphatase RQ is a phosphatidylinositol phosphatase that can regulate cell survival and proliferation. Proc. Natl. Acad. Sci. USA 100, 7563–7568 (2003).
Takenawa, T. & Itoh, T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta 1533, 190–206 (2001).
Kros, C.J. et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat. Neurosci. 5, 41–47 (2002).
Tilney, L.G., Tilney, M.S. & Cotanche, D.A. Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J. Cell Biol. 106, 355–365 (1988).
Verpy, E. et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat. Genet. 26, 51–55 (2000).
Di Palma, F. et al. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc. Natl. Acad. Sci. USA 99, 14994–14999 (2002).
Grimm, C. et al. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc. Natl. Acad. Sci. USA 104, 19583–19588 (2007).
Nagata, K. et al. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc. Natl. Acad. Sci. USA 105, 353–358 (2008).
van Aken, A.F. et al. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J. Physiol. (Lond.) 586, 5403–5418 (2008).
Maroto, R. et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 7, 179–185 (2005).
Gottlieb, P. et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 455, 1097–1103 (2008).
Shin, J.B. et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron 53, 371–386 (2007).
Holt, J.R. et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002).
Stauffer, E.A. et al. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron 47, 541–553 (2005).
Verpy, E. et al. Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nat. Genet. 29, 345–349 (2001).
Legan, P.K. et al. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 28, 273–285 (2000).
He, D.Z., Jia, S. & Dallos, P. Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 429, 766–770 (2004).
Chan, D.K. & Hudspeth, A.J. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat. Neurosci. 8, 149–155 (2005).
Mederos y Schnitzler, M. et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 27, 3092–3103 (2008).
Farge, E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 13, 1365–1377 (2003).
Petit, C., Levilliers, J. & Hardelin, J.-P. Molecular genetics of hearing loss. Annu. Rev. Genet. 35, 589–646 (2001).
Kros, C.J., Rüsch, A. & Richardson, G.P. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc. Roy. Soc. Lond. B 249, 185–193 (1992).
Acknowledgements
The authors' research was supported by grants from European Commission FP6 Integrated Project EuroHear LSHG-CT-2004-512063 (C.P. & G.R.), Fondation Raymonde et Guy Strittmatter (C.P.), Usher FAUN Stiftung (C.P.), Ernst-Jung Stiftung für Medizin (C.P.), Louis-Jeantet for Medicine Foundation (C.P.) and the Wellcome Trust (G.R.). The authors would like to thank V. Michel and R. Goodyear for their help in preparing the figures, A. Forge for the scanning electron micrographs in Figure 1, J. Levilliers for help in the preparation of the document and J.-P. Hardelin for criticism of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Petit, C., Richardson, G. Linking genes underlying deafness to hair-bundle development and function. Nat Neurosci 12, 703–710 (2009). https://doi.org/10.1038/nn.2330
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2330
This article is cited by
-
The genetic and phenotypic landscapes of Usher syndrome: from disease mechanisms to a new classification
Human Genetics (2022)
-
Central auditory deficits associated with genetic forms of peripheral deafness
Human Genetics (2022)
-
Expression pattern of cochlear microRNAs in the mammalian auditory hindbrain
Cell and Tissue Research (2021)
-
A Truncating GPSM2 Mutation Causes Autosomal Recessive Nonsyndromic Hearing Loss: a Case Report
SN Comprehensive Clinical Medicine (2021)
-
Interaction of protocadherin-15 with the scaffold protein whirlin supports its anchoring of hair-bundle lateral links in cochlear hair cells
Scientific Reports (2020)