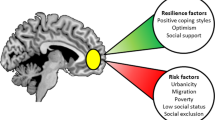
Abstract
Mental health and social life are intimately inter-related, as demonstrated by the frequent social deficits of psychiatric patients and the increased rate of psychiatric disorders in people exposed to social environmental adversity. Here, we review emerging evidence that combines epidemiology, social psychology and neuroscience to bring neural mechanisms of social risk factors for mental illness into focus. In doing so, we discuss existing evidence on the effects of common genetic risk factors in social neural pathways and outline the need for integrative approaches to identify the converging mechanisms of social environmental and genetic risk in brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
van Os, J., Rutten, B.P. & Poulton, R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr. Bull. 34, 1066–1082 (2008).
Penn, D.L., Sanna, L.J. & Roberts, D.L. Social cognition in schizophrenia: an overview. Schizophr. Bull. 34, 408–411 (2008).
Martin, D.J., Garske, J.P. & Davis, M.K. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J. Consult. Clin. Psychol. 68, 438–450 (2000).
Adolphs, R. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716 (2009).
Meyer-Lindenberg, A. & Weinberger, D.R. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat. Rev. Neurosci. 7, 818–827 (2006).
Heim, C. & Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 233, 102–111 (2012).
Krabbendam, L. & van Os, J. Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr. Bull. 31, 795–799 (2005).
Pedersen, C.B. & Mortensen, P.B. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch. Gen. Psychiatry 58, 1039–1046 (2001).
Bourque, F., van der Ven, E. & Malla, A. A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychol. Med. 41, 897–910 (2011).
Zammit, S. et al. Individuals, schools, and neighborhood: a multilevel longitudinal study of variation in incidence of psychotic disorders. Arch. Gen. Psychiatry 67, 914–922 (2010).
Pruessner, J.C., Champagne, F., Meaney, M.J. & Dagher, A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 24, 2825–2831 (2004).
McGowan, P.O. et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 12, 342–348 (2009).
Lederbogen, F. et al. City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501 (2011).
Selten, J.P. & Cantor-Graae, E. Hypothesis: social defeat is a risk factor for schizophrenia? Br. J. Psychiatry Suppl. 51, s9–s12 (2007).
Zink, C.F. et al. Know your place: neural processing of social hierarchy in humans. Neuron 58, 273–283 (2008).
Knafo, A. & Plomin, R. Prosocial behavior from early to middle childhood: genetic and environmental influences on stability and change. Dev. Psychol. 42, 771–786 (2006).
Caspi, A. et al. Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854 (2002).
Tost, H., Bilek, E. & Meyer-Lindenberg, A. Brain connectivity in psychiatric imaging genetics. Neuroimage doi:10.1016/j.neuroimage.2011.11.007 (9 November 2011).
Meyer-Lindenberg, A., Domes, G., Kirsch, P. & Heinrichs, M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12, 524–538 (2011).
Kirsch, P. et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493 (2005).
Viviani, D. et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107 (2011).
Zink, C.F., Stein, J.L., Kempf, L., Hakimi, S. & Meyer-Lindenberg, A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J. Neurosci. 30, 7017–7022 (2010).
Heim, C. et al. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol. Psychiatry 14, 954–958 (2009).
Bakermans-Kranenburg, M.J. & van Ijzendoorn, M.H. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc. Cogn. Affect. Neurosci. 3, 128–134 (2008).
Tost, H. et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc. Natl. Acad. Sci. USA 107, 13936–13941 (2010).
Wu, S. et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol. Psychiatry 58, 74–77 (2005).
Chen, F.S. et al. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc. Natl. Acad. Sci. USA 108, 19937–19942 (2011).
Thompson, R.J., Parker, K.J., Hallmayer, J.F., Waugh, C.E. & Gotlib, I.H. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology 36, 144–147 (2011).
Tost, H. et al. Neurogenetic effects of OXTR rs2254298 in the extended limbic system of healthy Caucasian adults. Biol. Psychiatry 70, e37–e39; author reply e41–e42 (2011).
Inoue, H. et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol. Psychiatry 68, 1066–1072 (2010).
Hammock, E.A. & Young, L.J. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308, 1630–1634 (2005).
Walum, H. et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc. Natl. Acad. Sci. USA 105, 14153–14156 (2008).
Meyer-Lindenberg, A. et al. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol. Psychiatry 14, 968–975 (2009).
Kurth, F. et al. Diminished gray matter within the hypothalamus in autism disorder: a potential link to hormonal effects? Biol. Psychiatry 70, 278–282 (2011).
Gillis, R.F. & Rouleau, G.A. The ongoing dissection of the genetic architecture of autistic spectrum disorder. Mol. Autism 2, 12 (2011).
Buckholtz, J.W. & Meyer-Lindenberg, A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 31, 120–129 (2008).
Coplan, J.D. et al. Early-life stress, corticotropin-releasing factor, and serotonin transporter gene: a pilot study. Psychoneuroendocrinology 36, 289–293 (2011).
Ressler, K.J. et al. Polymorphisms in CRHR1 and the serotonin transporter loci: gene × gene × environment interactions on depressive symptoms. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 153B, 812–824 (2010).
O'Donovan, M.C., Craddock, N.J. & Owen, M.J. Genetics of psychosis; insights from views across the genome. Hum. Genet. 126, 3–12 (2009).
Bigos, K.L. et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch. Gen. Psychiatry 67, 939–945 (2010).
Erk, S. et al. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch. Gen. Psychiatry 67, 803–811 (2010).
Mizrahi, R. et al. Increased stress-induced dopamine release in psychosis. Biol. Psychiatry 71, 561–567 (2012).
Armbruster, D. et al. Children under stress – COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. Int. J. Neuropsychopharmacol. doi:10.1017/S1461145711001763 (12 December 2011).
Corcoran, C.M. et al. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 135, 170–174 (2012).
Weinberger, D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669 (1987).
Miczek, K.A., Nikulina, E.M., Shimamoto, A. & Covington, H.E. III. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J. Neurosci. 31, 9848–9857 (2011).
Montague, P.R. et al. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–1164 (2002).
Szyf, M. The early life social environment and DNA methylation. Clin. Genet. doi:10.1111/j.1399-0004.2012.01843.x (13 February 2012).
Brennand, K.J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Anonymous. A decade for psychiatric disorders. Nature 463, 9 (2010).
Acknowledgements
A.M.-L. gratefully acknowledges grant support by Deutsche Forschungsgemeinschaft (SFB 636, KFO 256), German Federal Ministry of Education and Research (BMBF NGFN-MooDs, Bernstein-Programm funding number 01GQ1003B) and European Union (NEWMEDS, OPTIMIZE, EU-GEI, EU-AIMS) during the preparation of this manuscript. H.T. gratefully acknowledges grant support by the German Federal Ministry of Education and Research (BMBF 01GQ1102).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Meyer-Lindenberg, A., Tost, H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci 15, 663–668 (2012). https://doi.org/10.1038/nn.3083
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3083
This article is cited by
-
Genetic Variations in Elements of the Oxytocinergic Pathway are Associated with Attention/Hyperactivity Problems and Anxiety Problems in Childhood
Child Psychiatry & Human Development (2024)
-
Effects of NMDA-receptor blockade by ketamine on mentalizing and its neural correlates in humans: a randomized control trial
Scientific Reports (2023)
-
Blocking D2/D3 dopamine receptors in male participants increases volatility of beliefs when learning to trust others
Nature Communications (2023)
-
Sex Differences in Rats with the Valproate Model of Autism: Disturbances in Social Behavior and Changes in Drd1 Gene Expression in Various Brain Structures
Neuroscience and Behavioral Physiology (2023)
-
The neuroanatomy of social trust predicts depression vulnerability
Scientific Reports (2022)