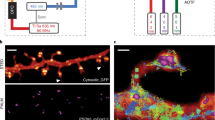
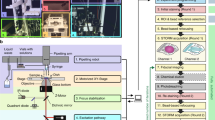
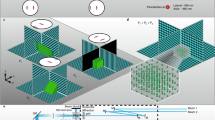
Abstract
Light microscopy can be applied in vivo and can sample large tissue volumes, features crucial for the study of single neurons and neural circuits. However, light microscopy per se is diffraction-limited in resolution, and the substructure of core signaling compartments of neuronal circuits—axons, presynaptic active zones, postsynaptic densities and dendritic spines—can be only insufficiently characterized by standard light microscopy. Recently, several forms of super-resolution light microscopy breaking the diffraction-imposed resolution limit have started to allow highly resolved, dynamic imaging in the cell-biologically highly relevant 10–100 nanometer range ('mesoscale'). New, sometimes surprising answers concerning how protein mobility and protein architectures shape neuronal communication have already emerged. Here we start by briefly introducing super-resolution microscopy techniques, before we describe their use in the analysis of neuronal compartments. We conclude with long-term prospects for super-resolution light microscopy in the molecular and cellular neurosciences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sigrist, S.J. & Sabatini, B.L. Optical super-resolution microscopy in neurobiology. Curr. Opin. Neurobiol. 22, 86–93 (2012).
Huang, B., Bates, M. & Zhuang, X. Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78, 993–1016 (2009).
Huang, B., Babcock, H. & Zhuang, X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143, 1047–1058 (2010).
Hell, S.W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Toomre, D. & Bewersdorf, J. A new wave of cellular imaging. Annu. Rev. Cell Dev. Biol. 26, 285–314 (2010).
Schermelleh, L., Heintzmann, R. & Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 190, 165–175 (2010).
Gustafsson, M.G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Schermelleh, L. et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332–1336 (2008).
Fiolka, R., Shao, L., Rego, E.H., Davidson, M.W. & Gustafsson, M.G. Time-lapse two-color 3D imaging of live cells with doubled resolution using structured illumination. Proc. Natl. Acad. Sci. USA 109, 5311–5315 (2012).
Hell, S.W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Meyer, L. et al. Dual-color STED microscopy at 30-nm focal-plane resolution. Small 4, 1095–1100 (2008).
Tønnesen, J. & Nagerl, U.V. Superresolution imaging for neuroscience. Exp. Neurol. 242, 33–40 (2013).
Hess, S.T., Girirajan, T.P. & Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, 4258–4272 (2006).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Rust, M.J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 (2006).
Patterson, G.H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Patterson, G., Davidson, M., Manley, S. & Lippincott-Schwartz, J. Superresolution imaging using single-molecule localization. Annu. Rev. Phys. Chem. 61, 345–367 (2010).
Bates, M., Huang, B., Dempsey, G.T. & Zhuang, X. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science 317, 1749–1753 (2007).
Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. Engl. 47, 6172–6176 (2008).
York, A.G. et al. Resolution doubling in live, multicellular organisms via multifocal structured illumination microscopy. Nat. Methods 9, 749–754 (2012).
Hein, B., Willig, K.I. & Hell, S.W. Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc. Natl. Acad. Sci. USA 105, 14271–14276 (2008).
Jones, S.A., Shim, S.H., He, J. & Zhuang, X. Fast, three-dimensional super-resolution imaging of live cells. Nat. Methods 8, 499–508 (2011).
Westphal, V. et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 320, 246–249 (2008).
Bückers, J., Wildanger, D., Vicidomini, G., Kastrup, L. & Hell, S.W. Simultaneous multi-lifetime multi-color STED imaging for colocalization analyses. Opt. Express 19, 3130–3143 (2011).
Lichtman, J.W. & Denk, W. The big and the small: challenges of imaging the brain's circuits. Science 334, 618–623 (2011).
Arenkiel, B.R. & Ehlers, M.D. Molecular genetics and imaging technologies for circuit-based neuroanatomy. Nature 461, 900–907 (2009).
Arenkiel, B.R. et al. Activity-induced remodeling of olfactory bulb microcircuits revealed by monosynaptic tracing. PLoS ONE 6, e29423 (2011).
Anderson, J.R. et al. A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 7, e1000074 (2009).
Anderson, J.R. et al. Exploring the retinal connectome. Mol. Vis. 17, 355–379 (2011).
Micheva, K.D. & Smith, S.J. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55, 25–36 (2007).
Lakadamyali, M., Babcock, H., Bates, M., Zhuang, X. & Lichtman, J. 3D multicolor super-resolution imaging offers improved accuracy in neuron tracing. PLoS ONE 7, e30826 (2012).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Xu, K., Zhong, G. & Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
Saka, S. & Rizzoli, S.O. Super-resolution imaging prompts re-thinking of cell biology mechanisms: selected cases using stimulated emission depletion microscopy. Bioessays 34, 386–395 (2012).
Sieber, J.J. et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317, 1072–1076 (2007).
Bar-On, D. et al. Super-resolution imaging reveals the internal architecture of nano-sized syntaxin clusters. J. Biol. Chem. 287, 27158–27167 (2012).
van den Bogaart, G. et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 (2011).
Aoyagi, K. et al. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 280, 17346–17352 (2005).
Willig, K.I., Rizzoli, S.O., Westphal, V., Jahn, R. & Hell, S.W. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature 440, 935–939 (2006).
Hua, Y. et al. A readily retrievable pool of synaptic vesicles. Nat. Neurosci. 14, 833–839 (2011).
Schmidt, R. et al. Spherical nanosized focal spot unravels the interior of cells. Nat. Methods 5, 539–544 (2008).
Sochacki, K.A. et al. Imaging the post-fusion release and capture of a vesicle membrane protein. Nat Commun. 3, 1154 (2012).
Südhof, T.C. The presynaptic active zone. Neuron 75, 11–25 (2012).
Sigrist, S.J. & Schmitz, D. Structural and functional plasticity of the cytoplasmic active zone. Curr. Opin. Neurobiol. 21, 144–150 (2011).
Neher, E. & Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59, 861–872 (2008).
Jiao, W., Masich, S., Franzen, O. & Shupliakov, O. Two pools of vesicles associated with the presynaptic cytosolic projection in Drosophila neuromuscular junctions. J. Struct. Biol. 172, 389–394 (2010).
Kittel, R.J. et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312, 1051–1054 (2006).
Wagh, D.A. et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833–844 (2006).
Fouquet, W. et al. Maturation of active zone assembly by Drosophila Bruchpilot. J. Cell Biol. 186, 129–145 (2009).
Hallermann, S. et al. Naked dense bodies provoke depression. J. Neurosci. 30, 14340–14345 (2010).
Liu, K.S. et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science 334, 1565–1569 (2011).
Owald, D. et al. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J. Cell Biol. 188, 565–579 (2010).
Frank, T. et al. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron 68, 724–738 (2010).
Dani, A., Huang, B., Bergan, J., Dulac, C. & Zhuang, X. Superresolution imaging of chemical synapses in the brain. Neuron 68, 843–856 (2010).
Bosch, M. & Hayashi, Y. Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 22, 383–388 (2012).
Kwon, H.B. & Sabatini, B.L. Glutamate induces de novo growth of functional spines in developing cortex. Nature 474, 100–104 (2011).
Harnett, M.T., Makara, J.K., Spruston, N., Kath, W.L. & Magee, J.C. Synaptic amplification by dendritic spines enhances input cooperativity. Nature 491, 599–602 (2012).
Shim, S.H. et al. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. USA 109, 13978–13983 (2012).
Nägerl, U.V., Willig, K.I., Hein, B., Hell, S.W. & Bonhoeffer, T. Live-cell imaging of dendritic spines by STED microscopy. Proc. Natl. Acad. Sci. USA 105, 18982–18987 (2008).
Berning, S., Willig, K.I., Steffens, H., Dibaj, P. & Hell, S.W. Nanoscopy in a living mouse brain. Science 335, 551 (2012).
Urban, N.T., Willig, K.I., Hell, S.W. & Nagerl, U.V. STED nanoscopy of actin dynamics in synapses deep inside living brain slices. Biophys. J. 101, 1277–1284 (2011).
Manley, S. et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 (2008).
Frost, N.A., Shroff, H., Kong, H., Betzig, E. & Blanpied, T.A. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67, 86–99 (2010).
Tatavarty, V., Kim, E.J., Rodionov, V. & Yu, J. Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS ONE 4, e7724 (2009).
Izeddin, I. et al. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE 6, e15611 (2011).
Tatavarty, V., Das, S. & Yu, J. Polarization of actin cytoskeleton is reduced in dendritic protrusions during early spine development in hippocampal neuron. Mol. Biol. Cell 23, 3167–3177 (2012).
Gold, M.G. A frontier in the understanding of synaptic plasticity: solving the structure of the postsynaptic density. Bioessays 34, 599–608 (2012).
MacGillavry, H.D., Kerr, J.M. & Blanpied, T.A. Lateral organization of the postsynaptic density. Mol. Cell Neurosci. 48, 321–331 (2011).
Newpher, T.M. & Ehlers, M.D. Glutamate receptor dynamics in dendritic microdomains. Neuron 58, 472–497 (2008).
Triller, A. & Choquet, D. New concepts in synaptic biology derived from single-molecule imaging. Neuron 59, 359–374 (2008).
Cognet, L., Groc, L., Lounis, B. & Choquet, D. Multiple routes for glutamate receptor trafficking: surface diffusion and membrane traffic cooperate to bring receptors to synapses. Sci. STKE 2006, pe13 (2006).
Triller, A. & Choquet, D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move!. Trends Neurosci. 28, 133–139 (2005).
Choquet, D. Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur. J. Neurosci. 32, 250–260 (2010).
Heine, M. et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320, 201–205 (2008).
Kerr, J.M. & Blanpied, T.A. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. 32, 658–673 (2012).
Hoze, N. et al. Heterogeneity of AMPA receptor trafficking and molecular interactions revealed by superresolution analysis of live cell imaging. Proc. Natl. Acad. Sci. USA 109, 17052–17057 (2012).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Shtengel, G. et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl. Acad. Sci. USA 106, 3125–3130 (2009).
Juette, M.F. et al. Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat. Methods 5, 527–529 (2008).
Zhang, M. et al. Rational design of true monomeric and bright photoactivatable fluorescent proteins. Nat. Methods 9, 727–729 (2012).
van de Linde, S., Wolter, S., Heilemann, M. & Sauer, M. The effect of photoswitching kinetics and labeling densities on super-resolution fluorescence imaging. J. Biotechnol. 149, 260–266 (2010).
Lau, L., Lee, Y.L., Sahl, S.J., Stearns, T. & Moerner, W.E. STED microscopy with optimized labeling density reveals 9-fold arrangement of a centriole protein. Biophys. J. 102, 2926–2935 (2012).
Ries, J., Kaplan, C., Platonova, E., Eghlidi, H. & Ewers, H. A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nat. Methods 9, 582–584 (2012).
Opazo, F. et al. Aptamers as potential tools for super-resolution microscopy. Nat. Methods 9, 938–939 (2012).
Watanabe, S. et al. Protein localization in electron micrographs using fluorescence nanoscopy. Nat. Methods 8, 80–84 (2011).
Nanguneri, S., Flottmann, B., Horstmann, H., Heilemann, M. & Kuner, T. Three-dimensional, tomographic super-resolution fluorescence imaging of serially sectioned thick samples. PLoS ONE 7, e38098 (2012).
Rostaing, P. et al. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24, 3463–3474 (2006).
Rueckel, M., Mack-Bucher, J.A. & Denk, W. Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing. Proc. Natl. Acad. Sci. USA 103, 17137–17142 (2006).
Ji, N., Sato, T.R. & Betzig, E. Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex. Proc. Natl. Acad. Sci. USA 109, 22–27 (2012).
Gould, T.J., Burke, D., Bewersdorf, J. & Booth, M.J. Adaptive optics enables 3D STED microscopy in aberrating specimens. Opt. Express 20, 20998–21009 (2012).
Kner, P., Chhun, B.B., Griffis, E.R., Winoto, L. & Gustafsson, M.G. Super-resolution video microscopy of live cells by structured illumination. Nat. Methods 6, 339–342 (2009).
Gustafsson, M.G. et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94, 4957–4970 (2008).
Schmidt, R. et al. Mitochondrial cristae revealed with focused light. Nano Lett. 9, 2508–2510 (2009).
Wildanger, D., Medda, R., Kastrup, L. & Hell, S.W. A compact STED microscope providing 3D nanoscale resolution. J. Microsc. 236, 35–43 (2009).
Xu, K., Babcock, H.P. & Zhuang, X. Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat. Methods 9, 185–188 (2012).
Hess, S.T. et al. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc. Natl. Acad. Sci. USA 104, 17370–17375 (2007).
Wombacher, R. et al. Live-cell super-resolution imaging with trimethoprim conjugates. Nat. Methods 7, 717–719 (2010).
Shao, L., Kner, P., Rego, E.H. & Gustafsson, M.G. Super-resolution 3D microscopy of live whole cells using structured illumination. Nat. Methods 8, 1044–1046 (2011).
Bates, M., Dempsey, G.T., Chen, K.H. & Zhuang, X. Multicolor super-resolution fluorescence imaging via multi-parameter fluorophore detection. Chemphyschem 13, 99–107 (2012).
Wilmes, S. et al. Triple-color super-resolution imaging of live cells: resolving submicroscopic receptor organization in the plasma membrane. Angew. Chem. Int. Ed. Engl. 51, 4868–4871 (2012).
Acknowledgements
We would like to thank D. Choquet, T. Kuner and S. Smith for sharing unpublished data and for scientific discussions. We thank T.A. Blanpied, H. Ewers, S. Hell, V. Nägerl, O. Shupliakov and X. Zhuang for permission to use their work. The authors are supported by grants from the Deutsche Forschungsgemeinschaft (TP A3/SFB 958; SI849 7-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Maglione, M., Sigrist, S. Seeing the forest tree by tree: super-resolution light microscopy meets the neurosciences. Nat Neurosci 16, 790–797 (2013). https://doi.org/10.1038/nn.3403
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3403
This article is cited by
-
Neurexin-3 subsynaptic densities are spatially distinct from Neurexin-1 and essential for excitatory synapse nanoscale organization in the hippocampus
Nature Communications (2023)
-
Hyperbolic material enhanced scattering nanoscopy for label-free super-resolution imaging
Nature Communications (2022)
-
Nuclear lamina invaginations are not a pathological feature of C9orf72 ALS/FTD
Acta Neuropathologica Communications (2021)
-
Super-resolution microscopy: a closer look at synaptic dysfunction in Alzheimer disease
Nature Reviews Neuroscience (2021)
-
Endo-microscopy beyond the Abbe and Nyquist limits
Light: Science & Applications (2020)