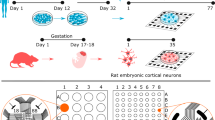
Abstract
This protocol describes a method for making and culturing rat hippocampal organotypic slices on membrane inserts. Supplementary videos are included to demonstrate visually the different steps of the procedure. Cultured hippocampal slices has been increasingly used as a model for synaptic studies of the brain as they allow examination of mid to long term manipulations in a preparation where the gross cytoarchitecture of the hippocampus is preserved. Combining techniques such as molecular biology, electrophysiology and immunohistochemistry to study physiological or pathological processes can easily be applied to organotypic slices. The technique described here can be used to make organotypic slices from other parts of the brain, other rodent species and from a range of ages. This protocol can be completed in 3 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gahwiler, B.H. Organotypic cultures of neural tissue. Trends. Neurosci. 11, 484–489 (1988).
Gahwiler, B.H. Nerve cells in culture: the extraordinary discovery by Ross Granville Harrison. Brain. Res. Bull. 50, 343–344 (1999).
Harrison, R.G. Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med. 4, 140–143 (1907).
Harrison, R.G. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zoo. 142, 5–73 (1959).
Keshishian, H. Ross Harrison's “The outgrowth of the nerve fiber as a mode of protoplasmic movement”. J. Exp. Zool A Comp. Exp. Biol. 301, 201–203 (2004).
Gahwiler, B.H. Organotypic monolayer cultures of nervous tissue. J. Neurosci. Methods 4, 329–342 (1981).
Hogue, M.J. Human fetal brain cells in tissue cultures: their identification and motility. J. Exp. Zool. 106, 85–107 (2006).
Stoppini, L., Buchs, P.A. & Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182 (1991).
Gahwiler, B.H., Capogna, M., Debanne, D., McKinney, R.A. & Thompson, S.M. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 20, 471–477 (1997).
De Simoni, A., Griesinger, C.B. & Edwards, F.A. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J. Physiol. 550, 135–147 (2003).
Buchs, P.A. & Muller, D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc. Natl. Acad. Sci. USA 93, 8040–8045 (1996).
Debanne, D., Gahwiler, B.H. & Thompson, S.M. Cooperative interactions in the induction of long-term potentiation and depression of synaptic excitation between hippocampal CA3–CA1 cell pairs in vitro. Proc. Natl. Acad. Sci. USA 93, 11225–11230 (1996).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Collin, C., Miyaguchi, K. & Segal, M. Dendritic spine density and LTP induction in cultured hippocampal slices. J. Neurophysiol 77, 1614–1623 (1997).
Li, Z. et al. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc. Natl. Acad. Sci. USA 102, 6131–6136 (2005).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Barria, A. & Malinow, R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48, 289–301 (2005).
Leutgeb, J.K., Frey, J.U. & Behnisch, T. LTP in cultured hippocampal-entorhinal cortex slices from young adult (P25–30) rats. J. Neurosci. Methods 130, 19–32 (2003).
Glover, C.P., Bienemann, A.S., Heywood, D.J., Cosgrave, A.S. & Uney, J.B. Adenoviral-mediated, high-level, cell-specific transgene expression: a SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity. Mol. Ther. 5, 509–516 (2002).
Miyaguchi, K., Maeda, Y., Collin, C. & Sihag, R.K. Gene transfer into hippocampal slice cultures with an adenovirus vector driven by cytomegalovirus promoter: stable co-expression of green fluorescent protein and lacZ genes. Brain Res. Bull. 51, 195–202 (2000).
De Paola, V., Arber, S. & Caroni, P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat. Neurosci. 6, 491–500 (2003).
Stricker, C. in Neuroscience Methods (ed. Martin, R.) (Harwood Academic Publishers, Amsterdam, 1997).
Nagerl, U.V., Eberhorn, N., Cambridge, S.B. & Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767 (2004).
Galimberti, I. et al. Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by experience. Neuron 50, 749–763 (2006).
Raineteau, O., Rietschin, L., Gradwohl, G., Guillemot, F. & Gahwiler, B.H. Neurogenesis in hippocampal slice cultures. Mol. Cell. Neurosci. 26, 241–250 (2004).
Nikonenko, I. et al. Integrins are involved in synaptogenesis, cell spreading, and adhesion in the postnatal brain. Brain Res. Dev. Brain Res. 140, 185–194 (2003).
Linke, R., Heimrich, B. & Frotscher, M. Axonal regeneration of identified septohippocampal projection neurons in vitro. Neuroscience 68, 1–4 (1995).
Lundstrom, K. et al. Semliki Forest virus vectors: efficient vehicles for in vitro and in vivo gene delivery. FEBS Lett. 504, 99–103 (2001).
Lundstrom, K., Abenavoli, A., Malgaroli, A. & Ehrengruber, M.U. Novel Semliki Forest virus vectors with reduced cytotoxicity and temperature sensitivity for long-term enhancement of transgene expression. Mol. Ther. 7, 202–209 (2003).
Kakegawa, W., Tsuzuki, K., Yoshida, Y., Kameyama, K. & Ozawa, S. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur. J. Neurosci. 20, 101–110 (2004).
Ehrengruber, M.U. et al. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc. Natl. Acad. Sci. USA 96, 7041–7046 (1999).
Perez Velazquez, J.L., Frantseva, M.V. & Carlen, P.L. In vitro ischemia promotes glutamate-mediated free radical generation and intracellular calcium accumulation in hippocampal pyramidal neurons. J. Neurosci. 17, 9085–9094 (1997).
Newell, D.W., Barth, A., Papermaster, V. & Malouf, A.T. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J. Neurosci. 15, 7702–7711 (1995).
Schmidt, H. et al. Organotypic hippocampal cultures. A model of brain tissue damage in Streptococcus pneumoniae meningitis. J. Neuroimmunol. 113, 30–39 (2001).
Gianinazzi, C. et al. Apoptosis of hippocampal neurons in organotypic slice culture models: direct effect of bacteria revisited. J. Neuropathol. Exp. Neurol. 63, 610–617 (2004).
Naumann, T., Linke, R. & Frotscher, M. Fine structure of rat septohippocampal neurons: I. Identification of septohippocampal projection neurons by retrograde tracing combined with electron microscopic immunocytochemistry and intracellular staining. J. Comp. Neurol. 325, 207–218 (1992).
Coltman, B.W., Earley, E.M., Shahar, A., Dudek, F.E. & Ide, C.F. Factors influencing mossy fiber collateral sprouting in organotypic slice cultures of neonatal mouse hippocampus. J. Comp. Neurol. 362, 209–222 (1995).
Ghoumari, A.M. et al. Mifepristone (RU486) protects Purkinje cells from cell death in organotypic slice cultures of postnatal rat and mouse cerebellum. Proc. Natl. Acad. Sci. USA 100, 7953–7958 (2003).
Becq, H., Bosler, O., Geffard, M., Enjalbert, A. & Herman, J.P. Anatomical and functional reconstruction of the nigrostriatal system in vitro: selective innervation of the striatum by dopaminergic neurons. J. Neurosci. Res. 58, 553–566 (1999).
Oishi, Y., Baratta, J., Robertson, R.T. & Steward, O. Assessment of factors regulating axon growth between the cortex and spinal cord in organotypic co-cultures: effects of age and neurotrophic factors. J. Neurotrauma 21, 339–356 (2004).
Hilton, K.J., Bateson, A.N. & King, A.E. A model of organotypic rat spinal slice culture and biolistic transfection to elucidate factors that drive the preprotachykinin-A promoter. Brain Res. Brain. Res. Rev. 46, 191–203 (2004).
Takuma, H., Sakurai, M. & Kanazawa, I. In vitro formation of corticospinal synapses in an organotypic slice co-culture. Neuroscience 109, 359–370 (2002).
Gong, Q., Liu, W.L., Srodon, M., Foster, T.D. & Shipley, M.T. Olfactory epithelial organotypic slice cultures: a useful tool for investigating olfactory neural development. Int. J. Dev. Neurosci. 14, 841–852 (1996).
Molnar, Z. & Blakemore, C. Development of signals influencing the growth and termination of thalamocortical axons in organotypic culture. Exp. Neurol. 156, 363–393 (1999).
Letinic, K., Zoncu, R. & Rakic, P. Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649 (2002).
Dong, H.W. & Buonomano, D.V. A technique for repeated recordings in cortical organotypic slices. J. Neurosci. Methods 146, 69–75 (2005).
Krassioukov, A.V. et al. An in vitro model of neurotrauma in organotypic spinal cord cultures from adult mice. Brain Res. Brain Res. Protoc. 10, 60–68 (2002).
Tom, V.J., Doller, C.M., Malouf, A.T. & Silver, J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J. Neurosci. 24, 9282–9290 (2004).
Mtchedlishvili, Z. & Kapur, J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol. Pharmacol. 69, 564–575 (2006).
Lo, D.C., McAllister, A.K. & Katz, L.C. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 13, 1263–1268 (1994).
McAllister, A.K., Lo, D.C. & Katz, L.C. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803 (1995).
McAllister, A.K. Biolistic transfection of cultured organotypic brain slices. Methods Mol. Biol. 245, 197–206 (2004).
O'Brien, J.A., Holt, M., Whiteside, G., Lummis, S.C. & Hastings, M.H. Modifications to the hand-held gene gun: improvements for in vitro biolistic transfection of organotypic neuronal tissue. J. Neurosci. Methods 112, 57–64 (2001).
Rathenberg, J., Nevian, T. & Witzemann, V. High-efficiency transfection of individual neurons using modified electrophysiology techniques. J. Neurosci. Methods 126, 91–98 (2003).
Beach, R.L., Bathgate, S.L. & Cotman, C.W. Identification of cell types in rat hippocampal slices maintained in organotypic cultures. Brain Res. 255, 3–20 (1982).
Benediktsson, A.M., Schachtele, S.J., Green, S.H. & Dailey, M.E. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J. Neurosci. Methods 141, 41–53 (2005).
London, J.A., Biegel, D. & Pachter, J.S. Neurocytopathic effects of beta-amyloid-stimulated monocytes: a potential mechanism for central nervous system damage in Alzheimer disease. Proc. Natl. Acad. Sci. USA 93, 4147–4152 (1996).
Noraberg, J. et al. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr. Drug Targets CNS Neurol. Disord. 4, 435–452 (2005).
Noraberg, J., Kristensen, B.W. & Zimmer, J. Markers for neuronal degeneration in organotypic slice cultures. Brain Res. Brain Res. Protoc. 3, 278–290 (1999).
Kim, J.A. et al. Cytoskeleton disruption causes apoptotic degeneration of dentate granule cells in hippocampal slice cultures. Neuropharmacology 42, 1109–1118 (2002).
Moroni, F. et al. Poly(ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ. 8, 921–932 (2001).
Heinemann, U. et al. Cell death and metabolic activity during epileptiform discharges and status epilepticus in the hippocampus. Prog. Brain Res. 135, 197–210 (2002).
Heinemann, U. et al. Coupling of electrical and metabolic activity during epileptiform discharges. Epilepsia 43 (Suppl. 5): 168–173 (2002).
Kovacs, R. et al. Free radical-mediated cell damage after experimental status epilepticus in hippocampal slice cultures. J. Neurophysiol 88, 2909–2918 (2002).
Acknowledgements
We are grateful to Dr. Frances A. Edwards, Dr. Yukiko Goda and Professor David Attwell for use of laboratory equipment and facilities. We also thank Professor David Attwell for valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
De Simoni, A., MY Yu, L. Preparation of organotypic hippocampal slice cultures: interface method. Nat Protoc 1, 1439–1445 (2006). https://doi.org/10.1038/nprot.2006.228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.228
This article is cited by
-
p-tau Ser356 is associated with Alzheimer’s disease pathology and is lowered in brain slice cultures using the NUAK inhibitor WZ4003
Acta Neuropathologica (2024)
-
Acute brain slice elastic modulus decreases over time
Scientific Reports (2023)
-
Agathisflavone Modifies Microglial Activation State and Myelination in Organotypic Cerebellar Slices Culture
Journal of Neuroimmune Pharmacology (2022)
-
Tau assemblies do not behave like independently acting prion-like particles in mouse neural tissue
Acta Neuropathologica Communications (2021)
-
Chronic Oral Administration of Magnesium-L-Threonate Prevents Oxaliplatin-Induced Memory and Emotional Deficits by Normalization of TNF-α/NF-κB Signaling in Rats
Neuroscience Bulletin (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.