Abstract
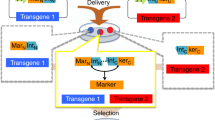
The first step in the generation of genetically tagged human embryonic stem cell (HESC) reporter lines is the isolation of cells that contain a stably integrated copy of the reporter vector. These cells are identified by their continued growth in the presence of a specific selective agent, usually conferred by a cassette encoding antibiotic resistance. In order to mitigate potential interference between the regulatory elements driving expression of the antibiotic resistance gene and those controlling the reporter gene, it is advisable to remove the positive selection cassette once the desired clones have been identified. This report describes a protocol for the removal of loxP-flanked selection cassettes from genetically modified HESCs by transient transfection with a vector expressing Cre recombinase. An integrated procedure for the clonal isolation of these genetically modified lines using single-cell deposition flow cytometry is also detailed. When performed sequentially, these protocols take ∼1 month.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Ng, E.S. et al. The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4-induced ventral mesoderm patterning in differentiating ES cells. Development 132, 873–884 (2005).
Gadue, P., Huber, T.L., Paddison, P.J. & Keller, G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA 103, 16806–16811 (2006).
Yasunaga, M. et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat. Biotechnol. 23, 1542–1550 (2005).
Micallef, S.J. et al. Endocrine cells develop within pancreatic bud-like structures derived from mouse ES cells differentiated in response to BMP4 and retinoic acid. Stem Cell Res. 1, 25–36 (2007).
Anderson, D. et al. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol. Ther. 15, 2027–2036 (2007).
Hedlund, E. et al. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells 25, 1126–1135 (2007).
Huber, I. et al. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 21, 2551–2563 (2007).
Davis, R.P. et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 111, 1876–1884 (2008).
Hug, B.A. et al. Analysis of mice containing a targeted deletion of beta-globin locus control region 5′ hypersensitive site 3. Mol. Cell. Biol. 16, 2906–2912 (1996).
Pham, C.T., MacIvor, D.M., Hug, B.A., Heusel, J.W. & Ley, T.J. Long-range disruption of gene expression by a selectable marker cassette. Proc. Natl. Acad. Sci. USA 93, 13090–13095 (1996).
Scacheri, P.C. et al. Bidirectional transcriptional activity of PGK-neomycin and unexpected embryonic lethality in heterozygote chimeric knockout mice. Genesis 30, 259–263 (2001).
Branda, C.S. & Dymecki, S.M. Talking about a revolution: review. The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004).
von Melchner, H. & Stewart, A.F. Engineering of ES cell genomes with recombinase systems. Methods Enzymol 420, 100–136 (2006).
Nolden, L. et al. Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nat. Methods 3, 461–467 (2006).
Taniguchi, M. et al. Efficient production of Cre-mediated site-directed recombinants through the utilization of the puromycin resistance gene, pac: a transient gene-integration marker for ES cells. Nucleic Acids Res. 26, 679–680 (1998).
Costa, M. et al. A method for genetic modification of human embryonic stem cells using electroporation. Nat. Protoc. 2, 792–796 (2007).
Ng, E.S., Davis, R., Stanley, E.G. & Elefanty, A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 3, 768–776 (2008).
Vallier, L., Alexander, M. & Pedersen, R. Conditional gene expression in human embryonic stem cells. Stem Cells 25, 1490–1497 (2007).
Thyagarajan, B. et al. Creation of engineered human embryonic stem cell lines using phiC31 integrase. Stem Cells 26, 119–126 (2007).
Costa, M., Sourris, K., Hatzistavrou, T., Elefanty, A.G. & Stanley, E.G. Expansion of human embryonic stem cells in vitro. Curr. Protoc. Stem Cell Biol. 1, 1C.1.1–1C.1.7 (2007).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Barnett, L.D. & Kontgen, F. Gene targeting in a centralized facility. Methods Mol. Biol. 158, 65–82 (2001).
Amit, M. et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278 (2000).
Xu, R.H. et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods 2, 185–190 (2005).
Köntgen, F. & Stewart, C.L. Simple screening procedure to detect gene targeting events in embryonic stem cells. Methods Enzymol. 225, 878–890 (1993).
Reubinoff, B.E., Pera, M.F., Vajta, G. & Trounson, A.O. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum. Reprod. 16, 2187–2194 (2001).
Acknowledgements
We thank Robyn Mayberry, Kathy Koutsis and Amanda Bruce for the provision of HESCs. This work was supported by the Australian Stem Cell Centre, the National Health and Medical Research Council of Australia (NHMRC) and the Juvenile Diabetes Research Foundation. A.G.E. is a senior research fellow of the NHMRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, R., Costa, M., Grandela, C. et al. A protocol for removal of antibiotic resistance cassettes from human embryonic stem cells genetically modified by homologous recombination or transgenesis. Nat Protoc 3, 1550–1558 (2008). https://doi.org/10.1038/nprot.2008.146
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.146
This article is cited by
-
Multipotent RAG1+ progenitors emerge directly from haemogenic endothelium in human pluripotent stem cell-derived haematopoietic organoids
Nature Cell Biology (2020)
-
Microhomology-assisted scarless genome editing in human iPSCs
Nature Communications (2018)
-
Genome Editing in Induced Pluripotent Stem Cells using CRISPR/Cas9
Stem Cell Reviews and Reports (2018)
-
NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network
Nature Communications (2018)
-
Long-term and efficient expression of human β-globin gene in a hematopoietic cell line using a new site-specific integrating non-viral system
Gene Therapy (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.