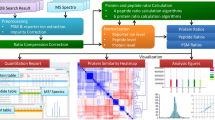
Abstract
In this paper, we describe the use of iTRAQ (isobaric Tags for Relative and Absolute Quantitation) tags for comparison of protein expression levels between multiple samples. These tags label all peptides in a protein digest before labeled samples are pooled, fractionated and analyzed using mass spectrometry (MS). As the tags are isobaric, the intensity of each peak is the sum of the intensity of this peptide from all samples, providing a moderate enhancement in sensitivity. On peptide fragmentation, amino-acid sequence ions also show this summed intensity, providing a sensitivity enhancement. However, the distinct distribution of isotopes in the tags is such that, on further fragmentation, a tag-specific reporter ion is released. The relative intensities of these ions represent the relative amount of peptide in the analytes. Integration of the relative quantification data for the peptides allows relative quantification of the protein. This protocol discusses the rationale behind design, optimization and performance of experiments, comparing protein samples using iTRAQ chemistries combined with strong cation exchange chromatographic fractionation and MS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Applied Biosystems. Applied Biosystems iTRAQ™ Reagents: Amine-Modifying Labeling Reagents for Multiplexed Relative and Absolute Protein Quantitation—Protocol (Applied Biosystems, Foster City, CA, 2004).
Applied Biosystems. iTRAQ® Reagents Chemical Reference Guide (Applied Biosystems, Foster City, CA, 2004).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003).
Ross, P.L. et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 (2004).
Hardt, M. et al. Assessing the effects of diurnal variation on the composition of human parotid saliva: quantitative analysis of native peptides using iTRAQ reagents. Anal. Chem. 77, 4947–4954 (2005).
Ogata, Y. et al. Differential protein expression in male and female human lumbar cerebrospinal fluid using iTRAQ reagents after abundant protein depletion. Proteomics 7, 3726–3734 (2007).
Kristiansson, M.H., Bhat, V.B., Babu, I.R., Wishnok, J.S. & Tannenbaum, S.R. Comparative time-dependent analysis of potential inflammation biomarkers in lymphoma-bearing SJL mice. J. Proteome Res. 6, 1735–1744 (2007).
DeSouza, L. et al. Search for cancer markers from endometrial tissues using differentially labeled tags iTRAQ and clCAT with multidimensional liquid chromatography and tandem mass spectrometry. J. Proteome Res. 4, 377–386 (2005).
Bouchal, P. et al. Biomarker discovery in low-grade breast cancer using isobaric stable isotope tags and two-dimensional liquid chromatography-tandem mass spectrometry (iTRAQ-2DLC-MS/MS) based quantitative proteomic analysis. J. Proteome Res. 8, 362–373 (2009).
Garbis, S.D. et al. Search for potential markers for prostate cancer diagnosis, prognosis and treatment in clinical tissue specimens using amine-specific isobaric tagging (iTRAQ) with two-dimensional liquid chromatography and tandem mass spectrometry. J. Proteome Res. 7, 3146–3158 (2008).
Unwin, R.D. et al. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood 107, 4687–4694 (2006).
Schnölzer, M., Jedrzejewski, P. & Lehmann, W.D. Protease-catalyzed incorporation of 18O into peptide fragments and its application for protein sequencing by electrospray and matrix-assisted laser desorption/ionization mass spectrometry. Electrophoresis 17, 945–953 (1996).
Faca, V. et al. Quantitative analysis of acrylamide labeled serum proteins by LC-MS/MS. J. Proteome Res. 5, 2009–2018 (2006).
Williamson, A.J.K. et al. Quantitative proteomics analysis demonstrates post-transcriptional regulation of embryonic stem cell differentiation to hematopoiesis. Mol. Cell. Proteomics 7, 459–472 (2008).
Lu, R. et al. Systems-level dynamic analysis of fate change in murine embryonic stem cells. Nature 462, 358–362 (2009).
Pierce, A. et al. Eight-channel iTRAQ enables comparison of the activity of six leukemogenic tyrosine kinases. Mol. Cell. Proteomics 7, 853–863 (2008).
Zhang, Y. et al. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Mol. Cell. Proteomics 4, 1240–1250 (2005).
Trinidad, J. Quantitative analysis of synaptic phosphorylation and protein expression. Mol. Cell. Proteomics 7, 684–696 (2007).
Wolf-Yadlin, A., Hautaniemi, S., Lauffenburger, D.A. & White, F.M. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc. Natl. Acad. Sci. USA 104, 5860–5865 (2007).
Wiese, S., Reidegeld, K.A., Meyer, H.E. & Warscheid, B. Protein labeling by iTRAQ: a new tool for quantitative mass spectrometry in proteome research. Proteomics 7, 340–350 (2007).
Karp, N.A. et al. Addressing accuracy and precision issues in iTRAQ quantitation. Mol. Cell Proteomics, published online (10 April 2010).
Ong, S.-E., Kratchmarova, I. & Mann, M. Properties of 13C-substituted arginine in stable isotope labelling by amino acids in cell culture (SILAC). J. Proteome Res. 2, 173–181 (2003).
Acknowledgements
This work is funded in part by the Leukaemia and Lymphoma Research Fund, UK, and by the Manchester NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
R.D.U. and J.R.G. acquired the data for setup experiments; R.D.U. analyzed the data; R.D.U., J.R.G. and A.D.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Data
Example Excel workbook describing steps in global QC of internal control samples in an iTRAQ experiment. The first sheet, entitled 'Control data', contains protein identification data exported from ProteinPilot along with ratio data for replicate channels for those proteins selected using the parameters described in the text. In column K, these data are converted into log2 space. Below the list (row 1059) shows the plot of log2(Ratio) against unused protein score. From this table it can be seen that 95% of the proteins (1003) lie between log2(ratios) -0.35 and 0.35 (ratio 0.785 and 1.275), suggesting that, in a test vs control experiment, ratios outside these limits are likely to be due to biological, rather than technical, variation. The second sheet, entitled 'Histograms' shows a frequency histogram of these data generated by the Excel Histogram tool, with the frequency of ratios which occur in a given 'bin' plotted as both a line plot and bar chart to show that the control data are approximately normally distributed and centred around 0. (XLS 436 kb)
Rights and permissions
About this article
Cite this article
Unwin, R., Griffiths, J. & Whetton, A. Simultaneous analysis of relative protein expression levels across multiple samples using iTRAQ isobaric tags with 2D nano LC–MS/MS. Nat Protoc 5, 1574–1582 (2010). https://doi.org/10.1038/nprot.2010.123
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.123
This article is cited by
-
Specific functions of single pistil S-RNases in S-gene homozygous Pyrus germplasm
BMC Plant Biology (2023)
-
Revealing the difference of α-amylase and CYP6AE76 gene between polyphagous Conogethes punctiferalis and oligophagous C. pinicolalis by multiple-omics and molecular biological technique
BMC Genomics (2022)
-
MAP2K6 remodels chromatin and facilitates reprogramming by activating Gatad2b-phosphorylation dependent heterochromatin loosening
Cell Death & Differentiation (2022)
-
iTRAQ-based proteomic analysis of sperm reveals candidate proteins that affect the quality of spermatozoa from boars on plateaus
Proteome Science (2021)
-
Quantitative proteomic analysis reveals that serine/threonine kinase is involved in Streptococcus suis virulence and adaption to stress conditions
Archives of Microbiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.