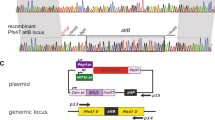
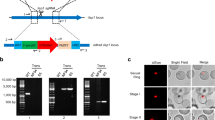
Abstract
The rodent malaria parasite Plasmodium chabaudi chabaudi shares many features with human malaria species, including P. falciparum, and is the in vivo model of choice for many aspects of malaria research in the mammalian host, from sequestration of parasitized erythrocytes, to antigenic variation and host immunity and immunopathology. This protocol describes an optimized method for the transformation of mature blood-stage P.c. chabaudi and a description of a vector that targets efficient, single crossover integration into the P.c. chabaudi genome. Transformed lines are reproducibly generated and selected within 14–20 d, and show stable long-term protein expression even in the absence of drug selection. This protocol, therefore, provides the scientific community with a robust and reproducible method to generate transformed P.c. chabaudi parasites expressing fluorescent, bioluminescent and model antigens that can be used in vivo to dissect many of the fundamental principles of malaria infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Langhorne, J., Quin, S.J. & Sanni, L.A. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chem. Immunol. 80, 204–228 (2002).
Stevenson, M.M. & Riley, E.M. Innate immunity to malaria. Nat. Rev. Immunol. 4, 169–180 (2004).
Reece, S.E., Duncan, A.B., West, S.A. & Read, A.F. Host cell preference and variable transmission strategies in malaria parasites. Proc. Biol. Sci. 272, 511–517 (2005).
de Roode, J.C. et al. Virulence and competitive ability in genetically diverse malaria infections. Proc. Natl. Acad. Sci. USA 102, 7624–7628 (2005).
Reece, S.E. & Thompson, J. Transformation of the rodent malaria parasite Plasmodium chabaudi and generation of a stable fluorescent line PcGFPCON. Malar. J. 7, 183 (2008).
Janse, C.J., Ramesar, J. & Waters, A.P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1, 346–356 (2006).
Sponaas, A.M. et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood 114, 5522–5531 (2009).
Belyaev, N.N. et al. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat. Immunol. 11, 477–485 (2010).
Franke-Fayard, B. et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137, 23–33 (2004).
Menard, R. & Janse, C. Gene targeting in malaria parasites. Methods 13, 148–157 (1997).
Acknowledgements
We thank B. Franke-Fayard (Leiden University Medical Centre, The Netherlands) for the kind gift of plasmid pL0017. This work was supported by the Medical Research Council (MRC; reference U117584248) and the Wellcome Trust (048684). P.J.S. is the recipient of a fellowship from the Leverhulme Trust, and J. Lawton is funded by an MRC PhD studentship.
Author information
Authors and Affiliations
Contributions
P.J.S., D.C., W.J., J. Langhorne and J.T. designed the experiments and wrote the manuscript. P.J.S., D.C., W.J. and J. Lawton performed the parasite purification, transfection optimization and parasite visualization experiments. J.T. designed and generated transfection plasmids.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Spence, P., Cunningham, D., Jarra, W. et al. Transformation of the rodent malaria parasite Plasmodium chabaudi. Nat Protoc 6, 553–561 (2011). https://doi.org/10.1038/nprot.2011.313
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.313
This article is cited by
-
Identification of gametocyte-associated pir genes in the rodent malaria parasite, Plasmodium chabaudi chabaudi AS
BMC Research Notes (2023)
-
ICAM-1 is a key receptor mediating cytoadherence and pathology in the Plasmodium chabaudi malaria model
Malaria Journal (2017)
-
Antibody-independent mechanisms regulate the establishment of chronic Plasmodium infection
Nature Microbiology (2017)
-
Signatures of malaria-associated pathology revealed by high-resolution whole-blood transcriptomics in a rodent model of malaria
Scientific Reports (2017)
-
Characterization of the Plasmodium Interspersed Repeats (PIR) proteins of Plasmodium chabaudi indicates functional diversity
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.