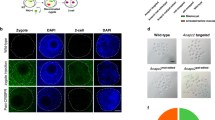
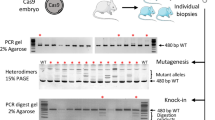
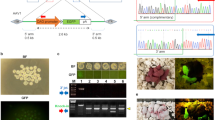
Abstract
Conventional embryonic stem cell (ESC)–based gene targeting, zinc-finger nuclease (ZFN) and transcription activator–like effector nuclease (TALEN) technologies are powerful strategies for the generation of genetically modified animals. Recently, the CRISPR/Cas system has emerged as an efficient and convenient alternative to these approaches. We have used the CRISPR/Cas system to generate rat strains that carry mutations in multiple genes through direct injection of RNAs into one-cell embryos, demonstrating the high efficiency of Cas9-mediated gene editing in rats for simultaneous generation of compound gene mutant models. Here we describe a stepwise procedure for the generation of knockout and knock-in rats. This protocol provides guidelines for the selection of genomic targets, synthesis of guide RNAs, design and construction of homologous recombination (HR) template vectors, embryo microinjection, and detection of mutations and insertions in founders or their progeny. The procedure from target design to identification of founders can take as little as 6 weeks, of which <10 d is actual hands-on working time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jacob, H.J., Lazar, J., Dwinell, M.R., Moreno, C. & Geurts, A.M. Gene targeting in the rat: advances and opportunities. Trends Genet. 26, 510–518 (2010).
Thomas, K.R. & Capecchi, M.R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51, 503–512 (1987).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Schwartzberg, P.L., Goff, S.P. & Robertson, E.J. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science 246, 799–803 (1989).
Mullins, L.J., Brooker, G. & Mullins, J.J. Transgenesis in the rat. Methods Mol. Biol. 180, 255–270 (2002).
Lois, C., Hong, E.J., Pease, S., Brown, E.J. & Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 (2002).
Zan, Y. et al. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat. Biotechnol. 21, 645–651 (2003).
Kitada, K. et al. Transposon-tagged mutagenesis in the rat. Nat. Methods 4, 131–133 (2007).
Katter, K. et al. Transposon-mediated transgenesis, transgenic rescue, and tissue-specific gene expression in rodents and rabbits. FASEB J. 27, 930–941 (2013).
Tong, C., Li, P., Wu, N.L., Yan, Y. & Ying, Q.L. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 467, 211–213 (2010).
Geurts, A.M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325, 433 (2009).
Cui, X. et al. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat. Biotechnol. 29, 64–67 (2011).
Tesson, L. et al. Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 29, 695–696 (2011).
Menoret, S. et al. Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB J. 27, 703–711 (2013).
Li, D. et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 31, 681–683 (2013).
Li, W., Teng, F., Li, T. & Zhou, Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol. 31, 684–686 (2013).
Hu, X. et al. Heritable gene-targeting with gRNA/Cas9 in rats. Cell Res. 23, 1322–1325 (2013).
Tong, C., Huang, G., Ashton, C., Li, P. & Ying, Q.L. Generating gene knockout rats by homologous recombination in embryonic stem cells. Nat. Protoc. 6, 827–844 (2011).
Men, H. & Bryda, E.C. Derivation of a germline competent transgenic Fischer 344 embryonic stem cell line. PloS ONE 8, e56518 (2013).
Men, H., Bauer, B.A. & Bryda, E.C. Germline transmission of a novel rat embryonic stem cell line derived from transgenic rats. Stem Cells Dev. 21, 2606–2612 (2012).
Hirabayashi, M. et al. Establishment of rat embryonic stem cell lines that can participate in germline chimerae at high efficiency. Mol. Reprod. Dev. 77, 94 (2010).
Hirabayashi, M. et al. Effect of leukemia inhibitory factor and forskolin on establishment of rat embryonic stem cell lines. J. Reprod. Dev. 60, 78–82 (2013).
Hirabayashi, M. et al. Rat transgenesis via embryonic stem cells electroporated with the Kusabira-orange gene. Mol. Reprod. Dev. 77, 474 (2010).
Bibikova, M., Beumer, K., Trautman, J.K. & Carroll, D. Enhancing gene targeting with designed zinc finger nucleases. Science 300, 764 (2003).
Miller, J.C. et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 25, 778–785 (2007).
Kim, Y.G., Cha, J. & Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 93, 1156–1160 (1996).
Mashimo, T. et al. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PloS ONE 5, e8870 (2010).
Porteus, M.H. & Baltimore, D. Chimeric nucleases stimulate gene targeting in human cells. Science 300, 763 (2003).
Qiu, Z. et al. High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. Nucleic Acids Res. 41, e120 (2013).
Wefers, B. et al. Generation of targeted mouse mutants by embryo microinjection of TALEN mRNA. Nat. Protoc. 8, 2355–2379 (2013).
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Moscou, M.J. & Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 (2009).
Sung, Y.H. et al. Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 31, 23–24 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Hwang, W.Y. et al. Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PloS ONE 8, e68708 (2013).
Cho, S.W., Kim, S., Kim, J.M. & Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Shen, B. et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723 (2013).
Garneau, J.E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Marraffini, L.A. & Sontheimer, E.J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463, 568–571 (2010).
Makarova, K.S. et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 9, 467–477 (2011).
Mali, P., Esvelt, K.M. & Church, G.M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963 (2013).
Sung, P. & Klein, H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat. Rev. Mol. Cell Biol. 7, 739–750 (2006).
Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79, 181–211 (2010).
Chen, F. et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods 8, 753–755 (2011).
Richardson, C., Moynahan, M.E. & Jasin, M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 12, 3831–3842 (1998).
Kim, E. et al. Precision genome engineering with programmable DNA-nicking enzymes. Genome Res. 22, 1327–1333 (2012).
Mashiko, D. et al. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 3, 3355 (2013).
Pattanayak, V. et al. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 31, 839–843 (2013).
Hsu, P.D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Yang, H. et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154, 1370–1379 (2013).
Fu, Y., Sander, J.D., Reyon, D., Cascio, V.M. & Joung, J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 (2014).
Ran, F.A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Xiao, A. et al. CasOT: a genome-wide Cas9/gRNA off-target searching tool. Bioinformatics 30, 1180–1182 (2014).
Hwang, W.Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Deng, C. & Capecchi, M.R. Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol. Cell. Biol. 12, 3365–3371 (1992).
Brown, A.J. et al. Whole-rat conditional gene knockout via genome editing. Nat. Methods 10, 638–640 (2013).
Ma, Y. et al. Generating rats with conditional alleles using CRISPR/Cas9. Cell Res. 24, 122–125 (2014).
Bryksin, A.V. & Matsumura, I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. BioTechniques 48, 463–465 (2010).
Mark, A. & Suckow, S.H.W.a.C.L.F. The Laboratory Rat 2nd edn. (Academic Press, 2006).
Brinster, R.L., Chen, H.Y., Trumbauer, M.E., Yagle, M.K. & Palmiter, R.D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl. Acad. Sci. USA 82, 4438–4442 (1985).
Mangerich, A. et al. A caveat in mouse genetic engineering: ectopic gene targeting in ES cells by bidirectional extension of the homology arms of a gene replacement vector carrying human PARP-1. Trans. Res. 18, 261–279 (2009).
Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. Manipulating the Mouse Embryo (Cold Spring Harbor Laboratory Press, 2003).
Si-Hoe, S.L., Wells, S. & Murphy, D. Production of transgenic rodents by the microinjection of cloned DNA into fertilized one-cell eggs. Mol. Biotechnol. 17, 151–182 (2001).
Acknowledgements
We thank F. Zhang of the Broad Institute of MIT and Harvard for kindly providing the Cas9 expression vector. We thank S. Siwko for comments and advice. This work was partially supported by grants from the State Key Development Programs of China (2010CB945403 to D.L. and 2012CB910400 to Mi.L.), grants from the National Natural Science Foundation of China (no. 31371455, 31171318 and 81330049) and a grant from the Science and Technology Commission of Shanghai Municipality (12XD1406100) and 14140900300.
Author information
Authors and Affiliations
Contributions
Y.S., Y.G., L. Wang, Z.Q., Me.L., Y.C., L. Wu and Y.L. performed the experiments; Y.S., Y.G., X.M., Mi.L. and D.L. analyzed the data; and Y.S., Y.G., Mi.L. and D.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 In vitro validation of sgRNA activity.
4 nM PCR product containing the target sequence was mixed with purified H6MBP-Cas9-4NLS recombinant protein (2 nM final concentration) in NEB buffer 3 with (lane 2) or without (lane 1) corresponding gRNAs for 1 hour. After the incubation, the protein was discarded and the DNA was analyzed by agarose gel electrophoresis.
Supplementary Figure 2 Design of PCR primers for genotyping of knock-in mutations in rat genome.
(a) A primer pair (F and R) is designed outside of the homology arms of the ssODN. (b) Two primer pairs (5'F/5'R and 3'F/3'R) are designed. For each pair, one primer is located in the GOI (the inserted fragment). The other primer is located in rat genome outside the homology arms (left arm or right arm). About 400 bp extension of both left and right arm of the donor plasmid can be used as the positive control for testing the primers (optional).
Supplementary Figure 3 Generation of double mutant rats by co-injection of Cas9/sgRNAs targeting the Fah and Il2rg loci.
Detection of mutations in F0 rats generated by injection of Cas9/sgRNAs targeting Fah (a) and Il2rg (b) after T7EI digestion using PCR products amplified from RNA-injected F0 rat tail genomic DNA. Double mutant rats are indicated by arrowheads. M, DNA marker. (c) DNA Sequence of Fah and Il2rg loci double mutant rats. The target sites are in blue with PAM in red.
Supplementary Figure 4 Anticipated results for precise knock-in by ssODNs.
(a) A diagram of Cas9 nickase induced precise gene editing. The sequence of the target site and the ssODNs are listed. (b) Typical sequences of the founders after RNA/DNA injection. The PCR products were amplified with the primers outside of the homology region indicated in (a). Sequences were determined by DNA sequencing of individual TA clones of PCR products. The sgRNA target is underlined, the EcoRI site is in red and the LoxP site is in italics. (c) An example of a DNA sequencing result for precise HR.
Supplementary information
Supplementary Figure 1
In vitro validation of sgRNA activity. (PDF 142 kb)
Supplementary Figure 2
Design of PCR primers for genotyping of knock-in mutations in rat genome. (PDF 243 kb)
Supplementary Figure 3
Generation of double mutant rats by co-injection of Cas9/sgRNAs targeting the Fah and Il2rg loci. (PDF 238 kb)
Supplementary Figure 4
Anticipated results for precise knock-in by ssODNs. (PDF 244 kb)
Supplementary Method 1
Preparation of recombinant Cas9 protein. (PDF 163 kb)
Supplementary Method 2
Functional validation of sgRNA and Cas9 protein in vitro. (PDF 98 kb)
Supplementary Table 1
Summary of injection statistics in rat embryos from published reports. (PDF 177 kb)
Supplementary Table 2
Primer or oligo sequences. (PDF 174 kb)
Pronuclear microinjection
This video demonstrates the microinjection of the CRISPR-Cas system into the pronucleus of a rat zygote fixed with a holding pipette on the left. An obvious pronuclear swelling is shown to demonstrate the successful pronuclear injection. (MOV 2515 kb)
Supplementary Data 1: Full DNA sequence of pGS3-T7-sgRNA vector for in vitro synthesis of gRNA.
The pair of reverse located BbsI restriction sites used for cloning is indicated in bold and underlined. The DraI restriction site used to linearize the plasmid for run-off transcription is shown in bold, italicized text. The “stuffer” sequence that will eventually be replaced with the target-coding oligonucleotides is highlighted in yellow and the “scaffold” portion of sgRNA is shown as italicized underlined text. The sequence of an M13 primer binding site is shown as underlined text. (PDF 208 kb)
Supplementary Data 2: Full DNA sequence of SP6-Cas9 vector for in vitro mRNA synthesis.
The SP6 promoter sequence (highlighted in green) is followed by a Kozak sequence (highlighted in yellow) is subcloned into the Cas9-containing plasmid pX260 (Addgene plasmid# 42229) using NcoI restriction site (in red), NLS-hCas9-NLS-coding cassette is underlined. (PDF 185 kb)
Rights and permissions
About this article
Cite this article
Shao, Y., Guan, Y., Wang, L. et al. CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos. Nat Protoc 9, 2493–2512 (2014). https://doi.org/10.1038/nprot.2014.171
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.171
This article is cited by
-
An analgesic pathway from parvocellular oxytocin neurons to the periaqueductal gray in rats
Nature Communications (2023)
-
Differences in renal cortex transcriptional profiling of wild-type and novel type B cystinuria model rats
Urolithiasis (2022)
-
Micropipette-based biomechanical nanotools on living cells
European Biophysics Journal (2022)
-
Fluorescence-coupled micropipette aspiration assay to examine calcium mobilization caused by red blood cell mechanosensing
European Biophysics Journal (2022)
-
Modification of improved-genome editing via oviductal nucleic acids delivery (i-GONAD)-mediated knock-in in rats
BMC Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.