Abstract
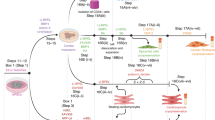
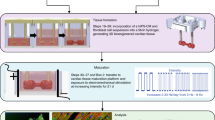
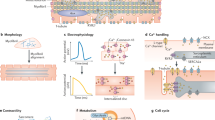
Since the advent of the generation of human induced pluripotent stem cells (hiPSCs), numerous protocols have been developed to differentiate hiPSCs into cardiomyocytes and then subsequently assess their ability to recapitulate the properties of adult human cardiomyocytes. However, hiPSC-derived cardiomyocytes (hiPSC-CMs) are often assessed in single-cell assays. A shortcoming of these assays is the limited ability to characterize the physiological parameters of cardiomyocytes, such as contractile force, due to random orientations. This protocol describes the differentiation of cardiomyocytes from hiPSCs, which occurs within 14 d. After casting, cardiomyocytes undergo 3D assembly. This produces fibrin-based engineered heart tissues (EHTs)—in a strip format—that generate force under auxotonic stretch conditions. 10–15 d after casting, the EHTs can be used for contractility measurements. This protocol describes parallel expansion of hiPSCs; standardized generation of defined embryoid bodies, growth factor and small-molecule-based cardiac differentiation; and standardized generation of EHTs. To carry out the protocol, experience in advanced cell culture techniques is required.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Burridge, P.W., Keller, G., Gold, J.D. & Wu, J.C. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10, 16–28 (2012).
Mercola, M., Colas, A. & Willems, E. Induced pluripotent stem cells in cardiovascular drug discovery. Circ. Res. 112, 534–548 (2013).
Knollmann, B.C. Induced pluripotent stem cell-derived cardiomyocytes: boutique science or valuable arrhythmia model? Circ. Res. 112, 969–976 (2013).
Hansen, A. et al. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 107, 35–44 (2010).
Schaaf, S. et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One 6, e26397 (2011).
Hirt, M.N. et al. Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res. Cardiol. 107, 307 (2012).
Mannhardt, I. et al. Human engineered heart tissue: analysis of contractile force. Stem Cell Rep. 7, 29–42 (2016).
Uzun, A.U. et al. Ca(2+)-currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions. Front. Pharmacol. 7, 300 (2016).
Weinberger, F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 8, 363ra148 (2016).
Thomson, J.A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Ludwig, T.E. et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 24, 185–187 (2006).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Xu, C. et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974 (2001).
Frank, S., Zhang, M., Scholer, H.R. & Greber, B. Small molecule-assisted, line-independent maintenance of human pluripotent stem cells in defined conditions. PLoS One 7, e41958 (2012).
Lanner, F. & Rossant, J. The role of FGF/Erk signaling in pluripotent cells. Development 137, 3351–3360 (2010).
Vallier, L., Alexander, M. & Pedersen, R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 118, 4495–4509 (2005).
Eiselleova, L. et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells 27, 1847–1857 (2009).
Ding, V.M. et al. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J. Cell Physiol. 225, 417–428 (2010).
Zoumaro-Djayoon, A.D. et al. Investigating the role of FGF-2 in stem cell maintenance by global phosphoproteomics profiling. Proteomics 11, 3962–3971 (2011).
Miltenyi Biotec. Human FGF-2 IS. <http://www.miltenyibiotec.com/en/products-and-services/macs-cell-culture-and-stimulation/cytokines-and-growth-factors/premium-and-research-grade/human-fgf-2-is.aspx.
Burridge, P.W. et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One 6, e18293 (2011).
Burridge, P.W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Laflamme, M.A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007).
Mummery, C. et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107, 2733–2740 (2003).
Kattman, S.J. et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240 (2011).
Moretti, A., Laugwitz, K.L., Dorn, T., Sinnecker, D. & Mummery, C. Pluripotent stem cell models of human heart disease. Cold Spring Harb. Perspect. Med. 3 http://dx.doi.org/10.1101/cshperspect.a014027 (2013).
Zweigerdt, R., Olmer, R., Singh, H., Haverich, A. & Martin, U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat. Protoc. 6, 689–700 (2011).
Olmer, R. et al. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng. Part C Methods 18, 772–784 (2012).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Kempf, H. et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 3, 1132–1146 (2014).
Kempf, H., Kropp, C., Olmer, R., Martin, U. & Zweigerdt, R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat. Protoc. 10, 1345–1361 (2015).
Chen, V.C. et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 15, 365–375 (2015).
Lanier, M. et al. Wnt inhibition correlates with human embryonic stem cell cardiomyogenesis: a structure-activity relationship study based on inhibitors for the Wnt response. J. Med. Chem. 55, 697–708 (2012).
Tulloch, N.L. et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 109, 47–59 (2011).
Kensah, G. et al. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur. Heart J. 34, 1134–1146 (2013).
Nunes, S.S. et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 10, 781–787 (2013).
Thavandiran, N. et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl. Acad. Sci. USA 110, E4698–4707 (2013).
Turnbull, I.C. et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 28, 644–654 (2014).
Hinson, J.T. et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349, 982–986 (2015).
Kuppusamy, K.T. et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc. Natl. Acad. Sci. USA 112, E2785–2794 (2015).
Stillitano, F. et al. Genomic correction of familial cardiomyopathy in human engineered cardiac tissues. Eur. Heart J. 37, 3282–3284 (2016).
Riegler, J. et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 117, 720–730 (2015).
Zhang, D. et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813–5820 (2013).
Wang, G. et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623 (2014).
Eschenhagen, T. et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 11, 683–694 (1997).
Zimmermann, W.H. et al. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 68, 106–114 (2000).
Zimmermann, W.H. et al. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 90, 223–230 (2002).
Neuber, C. et al. Paradoxical effects on force generation after efficient β1-adrenoceptor knockdown in reconstituted heart tissue. J. Pharmacol. Exp. Ther. 349, 39–46 (2014).
Stohr, A. et al. Contractile abnormalities and altered drug response in engineered heart tissue from Mybpc3-targeted knock-in mice. J. Mol. Cell. Cardiol. 63, 189–198 (2013).
Dussurget, O. & Roulland-Dussoix, D. Rapid, sensitive PCR-based detection of mycoplasmas in simulated samples of animal sera. Appl. Environ. Microbiol. 60, 953–959 (1994).
Ungrin, M.D., Joshi, C., Nica, A., Bauwens, C. & Zandstra, P.W. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One 3, e1565 (2008).
Kempf, H. et al. Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells. Nat. Commun. 7, 13602 (2016).
Lian, X., Zhang, J., Zhu, K., Kamp, T.J. & Palecek, S.P. Insulin inhibits cardiac mesoderm, not mesendoderm, formation during cardiac differentiation of human pluripotent stem cells and modulation of canonical Wnt signaling can rescue this inhibition. Stem Cells 31, 447–457 (2013).
Tran, T.H. et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells 27, 1869–1878 (2009).
Freund, C. et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells 26, 724–733 (2008).
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Eschenhagen, T., Eder, A., Vollert, I. & Hansen, A. Physiological aspects of cardiac tissue engineering. Am. J. Physiol. Heart Circ. Physiol. 303, H133–H143 (2012).
Lotz, S. et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS One 8, e56289 (2013).
Acknowledgements
We thank A. Moretti and K.-L. Laugwitz (University of Munich, Germany) for provision of the C25-hiPSC clone. This study was supported by the Deutsche Forschungsgemeinschaft (grants DFG Es 88/12-1 and DFG HA 3423/5-1), the British National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs CRACK-IT grant 35911-259146), the European Research Council (ERC-AG IndivuHeart), the EU (FP7 Biodesign), the German Centre for Cardiovascular Research (DZHK), the German Ministry of Education and Research (BMBF), British Heart Foundation grant RM/13/30157, the German Heart Foundation, the Freie und Hansestadt Hamburg and Era-Net E-RARE (grant 01GM1305).
Author information
Authors and Affiliations
Contributions
K.B. designed and performed the experiments, analyzed the data and wrote the paper. T.S., I.M., B.U., M.C.R., T.W., A.B., D.L.-B., B.K., M.P., G.M., S.L., A.S., D.S., S.F., C.N., E.K., U.S., M.L.S. and M.L.R. contributed to the development or validation of this protocol. A.H. supervised the project. A.H. and T.E. wrote and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
I.M., T.E. and A.H. are cofounders of EHT Technologies.
Integrated supplementary information
Supplementary Figure 1 Gating strategy used for FACS analysis of hiPSC-cardiomyocytes.
Given are details for forward and side scatter gates of the starting cell population and the gating to isolate cardiac troponin T-FITC-positive cells with the help of unstained cells (A) and cells stained with REA Control (I)-FITC isotype control (B). Absolute numbers of the cells analyzed and percentages of the relevant cell populations are provided. Cells were sorted with a BD FACSCanto II Flow Cytometer and analyzed with the BD FACSDiva Software.
Supplementary Figure 2 Gating strategy used for FACS analysis of hiPSCs.
Given are details for forward and side scatter gates of the starting cell population, the gating to discriminate doublets and aggregates and the gating to isolate TRA-1-60/SSEA-positive cells with the help of cells stained with isotype controls. Absolute numbers of the cells analyzed and percentages of the relevant cell populations are provided. Cells were sorted with a BD FACSCanto II Flow Cytometer and analyzed with the FlowJo Software.
Supplementary information
Supplementary Figures
Supplementary Figures 1 and 2. (PDF 693 kb)
Rights and permissions
About this article
Cite this article
Breckwoldt, K., Letuffe-Brenière, D., Mannhardt, I. et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat Protoc 12, 1177–1197 (2017). https://doi.org/10.1038/nprot.2017.033
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.033
This article is cited by
-
In situ monolayer patch clamp of acutely stimulated human iPSC-derived cardiomyocytes promotes consistent electrophysiological responses to SK channel inhibition
Scientific Reports (2024)
-
Remote-refocusing light-sheet fluorescence microscopy enables 3D imaging of electromechanical coupling of hiPSC-derived and adult cardiomyocytes in co-culture
Scientific Reports (2023)
-
Application of Human Induced Pluripotent Stem Cells for Tissue Engineered Cardiomyocyte Modelling
Regenerative Engineering and Translational Medicine (2023)
-
Modulating cardiac physiology in engineered heart tissue with the bidirectional optogenetic tool BiPOLES
Pflügers Archiv - European Journal of Physiology (2023)
-
An arrhythmogenic metabolite in atrial fibrillation
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.