Key Points
-
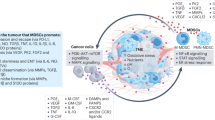
Myeloid cells can be abundant in the tumour stroma and emerging evidence indicates that the presence of these cells influences patient outcome in many cancer types.
-
Myeloid cells comprise various subsets that exhibit divergent functions. Whereas most myeloid cells promote cancer outgrowth, others display potent antitumour activity.
-
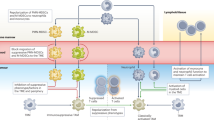
Tumours co-opt myeloid cells to promote cancer growth. This process occurs not only within the local tumour microenvironment but also in various distant body compartments.
-
Myeloid cells can favour or hinder each step of the metastatic cascade.
-
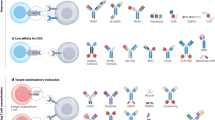
Myeloid cells have a central, yet still largely unexplored, role in virtually all therapeutic modalities, including surgery, chemotherapy, radiotherapy, immunotherapy and targeted therapy.
-
Modulating myeloid cell functions in therapy is an attractive option to augment the efficacy of current treatment options.
Abstract
Recent clinical trials have demonstrated the ability to durably control cancer in some patients by manipulating T lymphocytes. These immunotherapies are revolutionizing cancer treatment but benefit only a minority of patients. It is thus a crucial time for clinicians, cancer scientists and immunologists to determine the next steps in shifting cancer treatment towards better cancer control. This Review describes recent advances in our understanding of tumour-associated myeloid cells. These cells remain less studied than T lymphocytes but have attracted particular attention because their presence in tumours is often linked to altered patient survival. Also, experimental studies indicate that myeloid cells modulate key cancer-associated activities, including immune evasion, and affect virtually all types of cancer therapy. Consequently, targeting myeloid cells could overcome limitations of current treatment options.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Metchnikoff, E. Der Kampf der Phagocyten gegen Krankeitserreger. Virchows Arch. 96, 177–195 (1884).
Arandjelovic, S. & Ravichandran, K. S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16, 907–917 (2015).
Lavin, Y. et al. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 15, 731–744 (2015).
Steinman, R. M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Merad, M. et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
Broz, M. L. & Krummel, M. F. The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol. Res. 3, 313–319 (2015).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Gabrilovich, D. I. et al. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986).
Davies, L. C. et al. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277 (2012).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Pittet, M. J. et al. The journey from stem cell to macrophage. Ann. NY Acad. Sci. 1319, 1–18 (2014).
Diao, J. et al. Recruitment and differentiation of conventional dendritic cell precursors in tumors. J. Immunol. 184, 1261–1267 (2010).
Galli, S. J. et al. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 12, 1035–1044 (2011).
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006).
Rosenberg, H. F. et al. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22 (2013).
Lu, T. et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Invest. 121, 4015–4029 (2011).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472 (2015).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Ginhoux, F. et al. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 17, 34–40 (2016).
Pulford, K. A. et al. Distribution of the CD68 macrophage/myeloid associated antigen. Int. Immunol. 2, 973–980 (1990).
Kaiserling, E. et al. Aberrant expression of macrophage-associated antigens (CD68 and Ki-M1P) by Schwann cells in reactive and neoplastic neural tissue. Light- and electron-microscopic findings. Mod. Pathol. 6, 463–468 (1993).
Zhang, Q. W. et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE 7, e50946 (2012).
Steidl, C. et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N. Engl. J. Med. 362, 875–885 (2010). Detailed study showing decreased overall survival of patients whose tumours are infiltrated by macrophages.
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 5, 932–943 (2015).
Mohamed, M. M. & Sloane, B. F. Cysteine cathepsins: multifunctional enzymes in cancer. Nat. Rev. Cancer 6, 764–775 (2006).
Kessenbrock, K. et al. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Lewis, J. S. et al. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J. Pathol. 192, 150–158 (2000).
Sierra, J. R. et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J. Exp. Med. 205, 1673–1685 (2008).
Kuang, D. M. et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 206, 1327–1337 (2009).
Forssell, J. et al. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin. Cancer Res. 13, 1472–1479 (2007).
Ohno, S. et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 23, 5015–5022 (2003).
Ohno, S. et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 24, 3335–3342 (2004).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Squadrito, M. L. et al. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 34, 350–359 (2013).
Hibbs, J. B. et al. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235, 473–476 (1987).
Nathan, C. F. et al. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J. Exp. Med. 149, 100–113 (1979).
Urban, J. L. et al. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc. Natl Acad. Sci. USA 83, 5233–5237 (1986).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015). Meta-analysis of distinct leukocyte subsets posits neutrophils as significant predictors of poor patient survival for diverse solid tumour types.
Goc, J. et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 74, 705–715 (2014).
Dieu-Nosjean, M. C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Ladányi, A. et al. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 56, 1459–1469 (2007).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014). Identification of a discrete DC type in the tumour microenvironment that promotes antitumour immunity.
Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 4, 941–952 (2004).
Cubillos-Ruiz, J. R. et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538 (2015).
Spranger, S. et al. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015). Oncogenic β-catenin signalling prevents CD103+ DC recruitment to tumours, which limits tumour infiltration by T cells and sensitivity to immune checkpoint blockade.
Zelenay, S. et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015).
Ruffell, B. et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 (2014). The efficacy of M-CSF blockade can rely on suppressing IL-10 production by TAMs, which otherwise prevent antitumour DC and CD8+ T cell activation.
Treilleux, I. et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 10, 7466–7474 (2004).
Jensen, T. O. et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 118, 2476–2485 (2012).
Dzionek, A. et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165, 6037–6046 (2000).
Haniffa, M. et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37, 60–73 (2012).
Parker, K. H. et al. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv. Cancer Res. 128, 95–139 (2015).
Jiang, L. et al. Prognostic value of monocyte and neutrophils to lymphocytes ratio in patients with metastatic soft tissue sarcoma. Oncotarget 6, 9542–9550 (2015).
Huang, S. H. et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer 121, 545–555 (2015).
Gabitass, R. F. et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 60, 1419–1430 (2011).
Movahedi, K. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6Chigh monocytes. Cancer Res. 70, 5728–5739 (2010).
Sawanobori, Y. et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111, 5457–5466 (2008).
Cortez-Retamozo, V. et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl Acad. Sci. USA 109, 2491–2496 (2012).
Marvel, D. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 125, 3356–3364 (2015).
Vasquez-Dunddel, D. et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Invest. 123, 1580–1589 (2013).
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002).
Young, M. R. et al. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-β and nitric oxide. J. Immunol. 156, 1916–1922 (1996).
Mao, Y. et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 73, 3877–3887 (2013).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835 (2007).
Molon, B. et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208, 1949–1962 (2011).
Pan, P. Y. et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70, 99–108 (2010).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
Demers, M. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl Acad. Sci. USA 109, 13076–13081 (2012).
Wilson, C. L. et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 6, 6818 (2015).
Ortiz, M. L. et al. Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17-producing CD4+ T cells. J. Exp. Med. 212, 351–367 (2015).
Granot, Z. et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314 (2011).
Finisguerra, V. et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 522, 349–353 (2015).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009).
Eruslanov, E. B. et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Invest. 124, 5466–5480 (2014).
Davis, B. P. & Rothenberg, M. E. Eosinophils and cancer. Cancer Immunol. Res. 2, 1–8 (2014).
Nielsen, H. J. et al. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J. Pathol. 189, 487–495 (1999).
Tepper, R. I. et al. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257, 548–551 (1992).
Carretero, R. et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 16, 609–617 (2015). Eosinophils can contribute to tumour immunity by promoting tumour infiltration by T cells and polarizing TAMs.
Andreu, P. et al. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17, 121–134 (2010). Activating Fcγ receptors expressed by myeloid cells can foster a tumour-promoting microenvironment.
Nowak, E. C. et al. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J. Exp. Med. 209, 2127–2135 (2012).
Blatner, N. R. et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc. Natl Acad. Sci. USA 107, 6430–6435 (2010).
Gounaris, E. et al. Mast cells are an essential hematopoietic component for polyp development. Proc. Natl Acad. Sci. USA 104, 19977–19982 (2007).
Yang, Z. et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS ONE 5, e8922 (2010).
Saleem, S. J. et al. Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J. Immunol. 189, 511–515 (2012).
Malfettone, A. et al. High density of tryptase-positive mast cells in human colorectal cancer: a poor prognostic factor related to protease-activated receptor 2 expression. J. Cell. Mol. Med. 17, 1025–1037 (2013).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Väyrynen, J. P. et al. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br. J. Cancer 109, 1839–1847 (2013).
Katz, H. R. & Austen, K. F. Mast cell deficiency, a game of kit and mouse. Immunity 35, 668–670 (2011).
Feyerabend, T. B. et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity 35, 832–844 (2011).
Dudeck, A. et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 (2011).
Schönhuber, N. et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat. Med. 20, 1340–1347 (2014).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Franklin, R. A. et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Kitamura, T. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 212, 1043–1059 (2015).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Lin, E. Y. et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–740 (2001). Evidence from mice that M-CSF promotes mammary cancer metastasis.
Kubota, Y. et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 206, 1089–1102 (2009).
Richardsen, E. et al. Macrophage-colony stimulating factor (CSF1) predicts breast cancer progression and mortality. Anticancer Res. 35, 865–874 (2015).
Ueno, T. et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 6, 3282–3289 (2000).
Groblewska, M. et al. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin. Chem. Lab Med. 45, 30–34 (2007).
Mroczko, B. et al. Serum macrophage-colony stimulating factor levels in colorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin. Chim. Acta 380, 208–212 (2007).
Zhu, X. D. et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J. Clin. Oncol. 26, 2707–2716 (2008).
Smith, H. O. et al. The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin. Cancer Res. 1, 313–325 (1995).
Kirma, N. et al. Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-β1 in inducing c-fms expression. Cancer Res. 67, 1918–1926 (2007).
Pucci, F. et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle−B cell interactions. Science 352, 242–246 (2016). Macrophages in the subcapsular sinus of tumour-draining lymph nodes can suppress cancer outgrowth by blocking the spread of tumour-derived extracellular vesicles.
Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 209, 1135–1152 (2012).
Schiavoni, G. et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8α+ dendritic cells. J. Exp. Med. 196, 1415–1425 (2002).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Suzuki, S. et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8α− dendritic cell development. Proc. Natl Acad. Sci. USA 101, 8981–8986 (2004).
Cheong, C. et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209+ dendritic cells for immune T cell areas. Cell 143, 416–429 (2010).
Raccosta, L. et al. The oxysterol−CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med. 210, 1711–1728 (2013).
Casbon, A. J. et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl Acad. Sci. USA 112, E566–E575 (2015).
Bayne, L. J. et al. Tumor-derived granulocyte–macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835 (2012).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015). Neutrophils can suppress pulmonary and lymph node metastasis by suppressing antitumour T cell activity locally.
Talmadge, J. E. & Fidler, I. J. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 70, 5649–5669 (2010).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006).
Rohan, T. E. et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J. Natl Cancer Inst. 106, dju136 (2014).
Gül, N. et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Invest. 124, 812–823 (2014). Kupffer cells (liver macrophages) can phagocytose circulating tumour cells; antitumour antibodies that bind Fcγ receptors on Kupffer cells can trigger tumour cell elimination.
Willingham, S. B. et al. The CD47-signal regulatory protein α (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA 109, 6662–6667 (2012).
Steinert, G. et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 74, 1694–1704 (2014).
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010).
Hanna, R. N. et al. Patrolling monocytes control tumor metastasis to the lung. Science 350, 985–990 (2015). So-called patrolling monocytes are enriched in the lung tumour vasculature and prevent tumour cell invasion into the lungs by scavenging tumour material and recruiting NK cells.
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015). Neutrophils can promote tumour cell colonization of lung premetastatic niches by producing leukotrienes.
Spiegel, A. et al. Neutrophils suppress intraluminal NK-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 6, 630–649 (2016). Neutrophils can promote lung tumour metastasis by inhibiting NK cells and producing factors that facilitate tumour cell extravasation.
Sharma, S. K. et al. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J. Immunol. 194, 5529–5538 (2015).
Shree, T. et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 25, 2465–2479 (2011).
Xu, J. et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794 (2013).
Shiao, S. L. et al. TH2-polarized CD4+ T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol. Res. 3, 518–525 (2015).
DeNardo, D. G. et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 (2011). Targeting TAMs with an M-CSFR inhibitor substantially ameliorates chemotherapy against breast cancer in mice.
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Germano, G. et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23, 249–262 (2013).
Alizadeh, D. et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 74, 104–118 (2014).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Bonapace, L. et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515, 130–133 (2014).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013). Tumour irradiation with local low-dose ionizingradiation can stimulate TAM differentiation, which results in CD8+ T cell recruitment to the tumour site.
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). The microbiota can activate antitumour myeloid cell functions in response to chemotherapy.
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Kroemer, G. et al. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Ma, Y. et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38, 729–741 (2013).
Pfirschke, C. et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44, 343–354 (2016). Immunogenic chemotherapies can make tumours responsive to immune checkpoint blockade by activating antitumour myeloid cell functions.
Burnette, B. C. et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 71, 2488–2496 (2011).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Gupta, A. et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 189, 558–566 (2012).
Pincetic, A. et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 15, 707–716 (2014).
Bournazos, S. et al. The role of Fc–FcγR interactions in IgG-mediated microbial neutralization. J. Exp. Med. 212, 1361–1369 (2015).
Uchida, J. et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 199, 1659–1669 (2004).
Clynes, R. A. et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Overdijk, M. B. et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7, 311–321 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Simpson, T. R. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013).
Romano, E. et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA 112, 6140–6145 (2015). CTLA4 antibodies can eliminate T reg cells by engaging CD16+ monocytes; patients with melanoma who have more of these myeloid cells respond better to anti-CTLA4 therapy.
Li, F. & Ravetch, J. V. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science 333, 1030–1034 (2011).
Ahmad, Z. A. et al. scFv antibody: principles and clinical application. Clin. Dev. Immunol. 2012, 980250 (2012).
Curiel, T. J. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9, 562–567 (2003).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014). M-CSFR blockade can enhance antigen presentation, which augments antitumour T cell immunity and response to immune checkpoint blockade therapy.
Cavnar, M. J. et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J. Exp. Med. 210, 2873–2886 (2013).
Smith, M. P. et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFα. Cancer Discov. 4, 1214–1229 (2014).
Wang, T. et al. BRAF inhibition stimulates melanoma-associated macrophages to drive tumor growth. Clin. Cancer Res. 21, 1652–1664 (2015).
Mok, S. et al. Inhibition of colony stimulating factor-1 receptor improves antitumor efficacy of BRAF inhibition. BMC Cancer 15, 356 (2015).
Zeisberger, S. M. et al. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer 95, 272–281 (2006).
Gherardi, E. et al. Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer 12, 89–103 (2012).
Ott, P. A. et al. Inhibition of both BRAF and MEK in BRAFV600E mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol. Immunother. 62, 811–822 (2013).
Ott, P. A. & Bhardwaj, N. Impact of MAPK pathway activation in BRAFV600 melanoma on T cell and dendritic cell function. Front. Immunol. 4, 346 (2013).
Nefedova, Y. et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J. Immunol. 175, 4338–4346 (2005).
Cerny-Reiterer, S. et al. Long-term treatment with imatinib results in profound mast cell deficiency in Ph+ chronic myeloid leukemia. Oncotarget 6, 3071–3084 (2015).
Chow, A. et al. Studying the mononuclear phagocyte system in the molecular age. Nat. Rev. Immunol. 11, 788–798 (2011).
Srivastava, M. K. et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS ONE 7, e40677 (2012).
Ries, C. H. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014). Administration of an M-CSFR mAb substantially reduces TAM numbers and results in objective responses in patients with diffuse-type giant cell tumours.
Strachan, D. C. et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells. Oncoimmunology 2, e26968 (2013).
Mitchem, J. B. et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 (2013).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013). M-CSFR blockade functionally modulates, but does not deplete, TAMs and regresses established glioblastoma in mice.
Cortez-Retamozo, V. et al. Angiotensin II drives the production of tumor-promoting macrophages. Immunity 38, 296–308 (2013).
Petit, I. et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3, 687–694 (2002).
Cook, R. S. et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J. Clin. Invest. 123, 3231–3242 (2013).
Graham, D. K. et al. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 14, 769–785 (2014).
Nicodeme, E. et al. Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010).
Ivashkiv, L. B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 34, 216–223 (2013).
Wang, H. et al. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL-10. J. Immunol. 186, 3986–3996 (2011).
Chen, X. et al. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc. Natl Acad. Sci. USA 109, E2865–E2874 (2012).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011). Treatment with an agonist CD40 antibody can control cancer in mice by recruiting antitumour macrophages, independently of T cells.
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Hammerstrom, A. E. et al. Cancer immunotherapy: sipuleucel-T and beyond. Pharmacotherapy 31, 813–828 (2011).
Rauch, P. J. et al. Innate response activator B cells protect against microbial sepsis. Science 335, 597–601 (2012).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Barber, G. N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015).
Hanson, M. C. et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest. 125, 2532–2546 (2015).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl Med. 7, 283ra52 (2015). STING agonists can stimulate DCs to promote antitumour immunity and sensitivity to immune checkpoint blockade therapy.
Conlon, J. et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 190, 5216–5225 (2013).
Lemos, H. et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 76, 2076–2081 (2016).
Hubo, M. et al. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front. Immunol. 4, 82 (2013).
Shurin, G. V. et al. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J. Immunol. 183, 137–144 (2009).
Cubillos-Ruiz, J. R. et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 72, 1683–1693 (2012).
Yu, H. et al. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Ali, O. A. et al. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl Med. 1, 8ra19 (2009).
Bencherif, S. A. et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 6, 7556 (2015).
Kim, J. et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 33, 64–72 (2015).
Pittet, M. J. & Weissleder, R. Intravital imaging. Cell 147, 983–991 (2011).
Nakasone, E. S. et al. Imaging tumor–stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21, 488–503 (2012).
Leimgruber, A. et al. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia 11, 459–468 (2009).
Chittajallu, D. R. et al. In vivo cell-cycle profiling in xenograft tumors by quantitative intravital microscopy. Nat. Methods 12, 577–585 (2015).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Spitzer, M. H. et al. An interactive reference framework for modeling a dynamic immune system. Science 349, 1259425 (2015).
Calligaris, D. et al. Application of desorption electrospray ionization mass spectrometry imaging in breast cancer margin analysis. Proc. Natl Acad. Sci. USA 111, 15184–15189 (2014).
Ullal, A. V. et al. Cancer cell profiling by barcoding allows multiplexed protein analysis in fine-needle aspirates. Sci. Transl Med. 6, 219ra9 (2014).
Weissleder, R. et al. Imaging macrophages with nanoparticles. Nat. Mater. 13, 125–138 (2014).
Nahrendorf, M. et al. Hybrid PET-optical imaging using targeted probes. Proc. Natl Acad. Sci. USA 107, 7910–7915 (2010).
Bloy, N. et al. Trial watch: dendritic cell-based anticancer therapy. Oncoimmunology 3, e963424 (2014).
Lynch, T. J. et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 30, 2046–2054 (2012).
Wu, W. C. et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl Acad. Sci. USA 111, 4221–4226 (2014).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Van der Laan, A. M. et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 35, 376–385 (2014).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Bronte, V. & Pittet, M. J. The spleen in local and systemic regulation of immunity. Immunity 39, 806–818 (2013).
Kozin, S. V. et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 70, 5679–5685 (2010).
Tedder, T. F. et al. Fcγ receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Semin. Immunopathol. 28, 351–364 (2006).
Bulliard, Y. et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210, 1685–1693 (2013).
Beatty, G. L. et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6Clow F4/80+ extratumoral macrophages. Gastroenterology 149, 201–210 (2015).
Sluijter, M. et al. Inhibition of CSF-1R supports T-cell mediated melanoma therapy. PLoS ONE 9, e104230 (2014).
Rolny, C. et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19, 31–44 (2011).
Borg, C. et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Invest. 114, 379–388 (2004).
Chang, C. L. et al. Immune mechanism of the antitumor effects generated by bortezomib. J. Immunol. 189, 3209–3220 (2012).
Priceman, S. J. et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115, 1461–1471 (2010).
Zhang, W. et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin. Cancer Res. 16, 3420–3430 (2010).
Mok, S. et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 74, 153–161 (2014).
Acknowledgements
The authors thank members of the Pittet laboratory and of the Massachusetts General Hospital (MGH) Center for Systems Biology for critical discussions and acknowledge all contributors to the field whose work we could not cite owing to space limitations. This work was supported in part by the Samana Cay MGH Research Scholar Fund, National Institutes of Health (NIH) grants P50-CA86355, R21 CA190344 and R01-AI084880 (to M.J.P.), the Boehringer Ingelheim Fonds (to C.E.) and the Deutsche Forschungsgemeinschaft (DFG) PF809/1-1 (to C.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Innate immune system
-
A system comprising various cell types that together provide defence to the host against infection and injury and orchestrate inflammatory responses. Unlike adaptive immune cells, innate immune cells express only germline-encoded pattern recognition receptors and generally they do not provide long-lasting immunity; however, they can activate the adaptive immune system through a process known as antigen presentation.
- Macrophages
-
Differentiated cells of the mononuclear phagocyte lineage that can clear dead cells and foreign particles through a process called phagocytosis. Macrophages assume tissue- and microenvironment-specific phenotypes to regulate tissue homeostasis, immunity and inflammation; they are essential protectors against injury and infections but also contribute to many diseases, including cancer.
- Dendritic cells
-
(DCs). Crucial antigen-presenting cells for immune control. DCs typically have a probing morphology and localize in T cell areas of lymphoid organs to activate specific CD4+ and CD8+ T cells, but they can also be found in nonlymphoid tissues, such as the tumour stroma.
- Monocytes
-
Bone marrow-derived mononuclear phagocytes, crucial in protection against infections and in immune homeostasis, which when deployed to tissues can differentiate into a macrophage, and under certain conditions, a dendritic cell. Monocytes are typically divided into two subtypes: patrolling monocytes and inflammatory monocytes.
- Neutrophils
-
Polymorphonuclear cells that develop and mature in the bone marrow, exist at high numbers in circulation and can be rapidly recruited to a site of injury or inflammation. Neutrophils can release potent biologically active antimicrobial enzymes, which are directly involved in clearance of infection.
- Eosinophils
-
Granulocytic cells that are known mostly for their involvement in asthmatic disease and parasitic infections. Eosinophils are found primarily in the circulation, gut and thymic tissue but can be rapidly deployed into various tissues during inflammation to expel their granular content.
- Mast cells
-
Crucial innate effector cells that are rich in granules that contain various immunoregulatory molecules. Upon stimulation by pathogens, allergens or endogenous factors, mast cells can rapidly degranulate and profoundly affect local and systemic tissue homeostasis, as exemplified by anaphylaxis.
- Basophils
-
Circulating granulocytic cells known to mediate allergic responses and host defence against parasitic infections. Basophilic granules are a rich source of inflammatory mediators, including the vasodilator histamine and the anticoagulant heparin.
- Tertiary lymphoid structures
-
Ectopic lymph node-like arrangements that form in tissues under pathophysiological conditions and that seem to facilitate local lymphocyte activation.
- Regulatory T cells
-
(Treg cells). Specialized T cells that are functionally defined by their ability to confer peripheral tolerance to self, commensal and environmental antigens. Treg cell accumulation in tumours can suppress antitumour immunity and is associated with poor prognosis in many cancers.
- Degranulation
-
Release of cytotoxic and other molecules from secretory vesicles, also called granules, that are initially stored in some innate immune cells, for example, neutrophils, eosinophils and mast cells.
- M-CSF–M-CSFR
-
(macrophage-colony stimulating factor and its receptor, also known as CSF1–CSF1R). A haematopoietic growth factor–receptor pair that is required for proper development, survival and maintenance of the monocyte and macrophage cell lineage.
- CCL2–CCR2
-
(chemokine (C-C motif) ligand/receptor 2). A chemokine–receptor pair that mediates monocyte release from the bone marrow and, in the context of cancer, entry into the tumour microenvironment.
- Premetastatic sites
-
Sites in which metastasis will occur. These sites are thought to be primed for tumour cell engraftment by factors that are secreted by the primary tumour and by bone marrow-derived haematopoietic cells that are recruited locally.
- Natural killer (NK) cells
-
Cytotoxic lymphocytes that are crucial to the innate immune system and that provide rapid responses to eliminate abnormal cells, such as virus-infected cells and tumour cells.
- Fcγ-receptor
-
(FcγR). A surface-bound protein receptor expressed by phagocytes and other cell types, which binds to the constant heavy chain (Fc) region of an antibody and mediates cell clearance mechanisms. FcγRs, for which four different classes are known (FcγRI, FcγRII, FcγRIII and FcγRIV), bind to the Fc region of immunoglobulin G antibodies.
Rights and permissions
About this article
Cite this article
Engblom, C., Pfirschke, C. & Pittet, M. The role of myeloid cells in cancer therapies. Nat Rev Cancer 16, 447–462 (2016). https://doi.org/10.1038/nrc.2016.54
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.54
This article is cited by
-
The roles of tissue resident macrophages in health and cancer
Experimental Hematology & Oncology (2024)
-
FFAR2 expressing myeloid-derived suppressor cells drive cancer immunoevasion
Journal of Hematology & Oncology (2024)
-
Inactivation of pentraxin 3 suppresses M2-like macrophage activity and immunosuppression in colon cancer
Journal of Biomedical Science (2024)
-
Macrophages reprogramming improves immunotherapy of IL-33 in peritoneal metastasis of gastric cancer
EMBO Molecular Medicine (2024)
-
Understanding current experimental models of glioblastoma-brain microenvironment interactions
Journal of Neuro-Oncology (2024)