Key Points
-
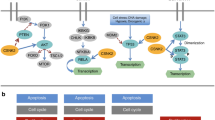
Signal transducer and activator of transcription (STAT)-family proteins are latent cytoplasmic transcription factors that convey signals from cytokine and growth-factor receptors to the nucleus.
-
STAT proteins, particularly STAT3 and the STAT5 proteins, are frequently overactivated in a variety of human solid tumours and blood malignancies.
-
Continuous deregulation of nuclear gene expression by persistent STAT3 and STAT5 signalling promotes the growth and survival of tumour cells, thereby contributing to malignancy.
-
Persistent STAT3 signalling in tumour cells induces tumour angiogenesis and suppresses anti-tumour immune responses, further enhancing tumour progression.
-
Tumour cells that become dependent on persistent STAT3 signalling are more sensitive to STAT3 inhibitors than normal cells, providing a therapeutic window based on transient or partial inhibition of STAT3.
-
Proof-of-concept studies in cell-culture and animal models have validated STAT3 and STAT5 proteins as promising molecular targets for novel cancer therapies, including small-molecule inhibitors of STAT signalling.
Abstract
Tumour cells acquire the ability to proliferate uncontrollably, resist apoptosis, sustain angiogenesis and evade immune surveillance. STAT proteins — especially STAT3 and STAT5 — regulate all of these processes and are persistently activated in a surprisingly large number of human cancers. Consequently, STAT proteins are emerging — unexpectedly — as ideal targets for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bishop, J. M. The molecular genetics of cancer. Science 235, 305–311 (1987).
Vogelstein, B. & Kinzler, K. W. The multistep nature of cancer. Trends Genet. 9, 138–141 (1993).
Hahn, W. C. & Weinberg, R. A. Rules for making human tumor cells. New England J. Med. 347, 1593–1603 (2002).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Darnell, J. E. Jr Transcription factors as targets for cancer therapy. Nature Rev. Cancer 2, 740–749 (2002). Superb review that makes a persuasive case for targeting transcription factors for cancer therapy.
Egan, S. E. & Weinberg, R. A. The pathway to signal achievement. Nature 365, 781–783 (1993).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Darnell, J. E. Jr, Kerr, I. M. & Stark, G. R. Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994).
Darnell, J. E. Jr STATs and gene regulation. Science 277, 1630–1635 (1997).
Bromberg, J. & Darnell, J. E. Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473 (2000).
Bowman, T., Garcia, R., Turkson, J. & Jove, R. STATs in oncogenesis. Oncogene 19, 2474–2488 (2000).
Darnell, J. E. Jr Studies of IFN-induced transcriptional activation uncover the Jak–Stat pathway. Interferon Cytokine Res. 18, 549–554 (1998).
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Levitzki, A. Protein tyrosine kinase inhibitors as novel therapeutic agents. Pharmacol. Ther. 82, 231–239 (1999).
Gibbs, J. B. Mechanism-based target identification and drug discovery in cancer research. Science 287, 1969–1973 (2000).
Buettner, R., Mora, L. B. & Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 8, 945–954 (2002).
Taga, T. & Kishimoto, T. gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15, 797–819 (1997).
Ihle, J. N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13, 211–217 (2001).
Reddy, E. P., Korapati, A., Chaturvedi, P. & Rane, S. IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled. Oncogene 19, 2532–2547 (2000).
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nature Rev. Mol. Cell Biol. 3, 651–662 (2002).
Hirano, T., Ishihara, K. & Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19, 2548–2556 (2000).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Silvennoinen, O., Schindler, C., Schlessinger, J. & Levy, D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science 261, 1736–1739 (1993).
Zhong, Z., Wen, Z. & Darnell, J. E. Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264, 95–98 (1994).
Ruff-Jamison, S. et al. Epidermal growth factor and lipopolysaccharide activate Stat3 transcription factor in mouse liver. J. Biol. Chem. 269, 21933–21935 (1994).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Parsons, J. T. & Parsons, S. J. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 9, 187–192 (1997).
Irby, R. B. & Yeatman, T. J. Role of Src expression and activation in human cancer. Oncogene 19, 5636–5642 (2000).
Danial, N. N. & Rothman, P. JAK-STAT signaling activated by Abl oncogenes. Oncogene 19, 2523–2531 (2000).
Lin, T. S., Mahajan, S. & Frank, D. A. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene 19, 2496–2504 (2000).
Yu, C. L. et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269, 81–83 (1995). First report of persistent activation of STAT3 signalling by an oncoprotein.
Garcia, R. et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 8, 1267–1276 (1997).
Bromberg, J. F., Horvath, C. M., Besser, D., Lathem, W. W. & Darnell, J. E. Jr Stat3 activation is required for cellular transformation by v-src. Mol. Cell. Biol. 18, 2553–2558 (1998). References 34 and 35 provide the first evidence for a requirement of activated STAT3 signalling in cell transformation by an oncoprotein.
Turkson, J. et al. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18, 2545–2552 (1998).
Bromberg, J. F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999). An activated STAT3 mutant provides genetic evidence for the intrinsic oncogenic potential of the STAT3 protein.
Grandis, J. R. et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth in vitro. J. Clin. Invest. 102, 1385–1392 (1998). Demonstration that STAT3 signalling is required for growth of human head and neck cancer cells.
Catlett-Falcone, R. et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115 (1999). The first direct evidence that inhibition of persistent STAT3 signalling in human tumour cells induces apoptosis.
Lou, W., Ni, Z., Dyer, K., Tweardy, D. J. & Gao, A. C. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate 42, 239–242 (2000).
Mora, L. B. et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 62, 6659–6666 (2002).
Song, J. I. & Grandis, J. R. STAT signaling in head and neck cancer. Oncogene 19, 2489–2495 (2000).
Hung, W. & Elliott, B. Cooperative effect of c-Src tyrosine kinase and Stat3 in activation of hepatocyte growth factor expression in mammary carcinoma cells. J. Biol. Chem. 276, 12395–12403 (2001).
Zhang, Y. W., Wang, L. M., Jove, R. & Vande Woude, G. F. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21, 217–226 (2002).
Garcia, R. et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20, 2499–2513 (2001).
Niu, G. et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 21, 7001–7010 (2002).
Song, L., Turkson, J., Karras, J. G., Jove, R. & Haura, E. B. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 22, 4150–4165 (2003).
Sriuranpong, V. et al. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 63, 2948–2956 (2003).
Xi, S. et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J. Biol. Chem. 278, 31574–31583 (2003).
Frank, D. A. STAT signaling in cancer: insights into pathogenesis and treatment strategies. Cancer Treat. Res. 115, 267–291 (2003).
Schwaller, J. et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol. Cell 6, 693–704 (2000).
Huang, M. et al. Inhibition of Bcr–Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells. Oncogene 21, 8804–8816 (2002).
Levis, M. et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood 99, 3885–3891 (2002).
Onishi, M. et al. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol. Cell. Biol. 18, 3871–3879 (1998).
Bromberg, J. F., Horvath, C. M., Wen, Z., Schreiber, R. D. & Darnell, J. E. Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon α and interferon γ. Proc. Natl Acad. Sci. USA 93, 7673–7678 (1996).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Catlett-Falcone, R., Dalton, W. S. & Jove, R. STAT proteins as novel targets for cancer therapy. Curr. Opin. Oncol. 11, 490–496 (1999).
Turkson, J. & Jove, R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene 19, 6613–6626 (2000).
Sillaber, C., Gesbert, F., Frank, D. A., Sattler, M. & Griffin, J. D. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood 95, 2118–2125 (2000).
Grandis, J. R. et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc. Natl Acad. Sci. USA 97, 4227–4232 (2000).
Gesbert, F. & Griffin, J. D. Bcr/Abl activates transcription of the Bcl-X gene through STAT5. Blood 96, 2269–2276 (2000).
Horita, M. et al. Blockade of the Bcr–Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J. Exp. Med. 191, 977–984 (2000).
Zamo, A. et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 21, 1038–1047 (2002).
Epling-Burnette, P. K. et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest. 107, 351–362 (2001).
Aoki, Y., Feldman, G. M. & Tosato, G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101, 1535–1542 (2003).
Shen, Y., Devgan, G., Darnell, J. E. Jr & Bromberg, J. F. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc. Natl Acad. Sci. USA 98, 1543–1548 (2001).
Dang, C. V. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 19, 1–11 (1999).
Prendergast, G. C. Mechanisms of apoptosis by c-Myc. Oncogene 18, 2967–2987 (1999).
Bowman, T. et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl Acad. Sci. USA 98, 7319–7324 (2001).
Ramana, C. V. et al. Regulation of c-myc expression by IFN-γ through Stat1-dependent and-independent pathways. EMBO J. 19, 263–272 (2000).
Martino, A., Holmes, J. H., Lord, J. D., Moon, J. J. & Nelson, B. H. Stat5 and Sp1 regulate transcription of the cyclin D2 gene in response to IL-2. J. Immunol. 166, 1723–1729 (2001).
Kijima, T. et al. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 13, 355–362 (2002).
Masuda, M. et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 62, 3351–3355 (2002).
Sinibaldi, D. et al. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19, 5419–5427 (2000).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Raman, V. et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature 405, 974–978 (2000).
Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl Cancer Inst. 82, 4–6 (1990).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Folkman, J. in Cancer Medicine. (eds. Holland, J. F. et al.) 181–204. (Williams and Wilkins, Baltimore, 1997).
Rak, J., Yu, J. L., Klement, G. & Kerbel, R. S. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J. Investig. Dermatol. Symp. Proc. 5, 24–33 (2000).
Grunstein, J., Roberts, W. G., Mathieu-Costello, O., Hanahan, D. & Johnson, R. S. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 59, 1592–1598 (1999).
Millauer, B., Shawver, L. K., Plate, K. H., Risau, W. & Ullrich, A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature 367, 576–579 (1994).
Plate, K. H., Breier, G., Weich, H. A. & Risau, W. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature 359, 845–848 (1992).
Veikkola, T. & Alitalo, K. VEGFs, receptors and angiogenesis. Semin. Cancer Biol. 9, 211–220 (1999).
Veikkola, T., Karkkainen, M., Claesson-Welsh, L. & Alitalo, K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 60, 203–212 (2000).
Niu, G. et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21, 2000–2008 (2002). First report that VEGF is a direct target gene of STAT3.
Wei, D. et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22, 319–329 (2003).
Wei, L. H. et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22, 1517–1527 (2003).
Semenza, G. L. Involvement of hypoxia-inducible factor 1 in human cancer. Intern. Med. 41, 79–83 (2002).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Ravi, R. et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1 α. Genes Dev. 14, 34–44 (2000).
Bartoli, M. et al. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 17, 1562–1564 (2003).
Yahata, Y. et al. Nuclear translocation of phosphorylated STAT3 is essential for VEGF-induced human dermal microvascular endothelial cell migration and tube formation. J. Biol. Chem. 278, 40026–40031 (2003).
Deo, D. D. et al. Phosphorylation of Stat-3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet-activating factor, JAK-2, and Src in human umbilical vein endothelial cells: evidence for a dual kinase mechanism. J. Biol. Chem. 277, 21237–21245 (2002).
Pardoll, D. M. Spinning molecular immunology into successful immunotherapy. Nature Rev. Immunol. 2, 227–238 (2002).
Pardoll, D. M. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21, 807–839 (2003). References 94 and 95 provide comprehensive reviews on the current understanding of the mechanisms of tumour immune evasion.
Takeda, K. et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999).
Lee, C. K. et al. Stat3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity 17, 63–72 (2002).
Welte, T. et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc. Natl Acad. Sci. USA 100, 1879–1884 (2003).
Wang, T. et al. Regulation of the innate and adaptive immune responses by Stat3 signaling in tumor cells. Nature Med. 10, 48–54 (2004). Demonstration that persistent STAT3 activity in tumour cells suppresses anti-tumour immune responses.
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Med. 2, 1096–1103 (1996).
Yang, A. S. & Lattime, E. C. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 63, 2150–2157 (2003).
Ratta, M. et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 100, 230–237 (2002).
Sombroek, C. C. et al. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J. Immunol. 168, 4333–4343 (2002).
Nefedova, Y. et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 172, 464–474 (2004).
Cheng, F. et al. A critical role for Stat3 signaling in immune tolerance. Immunity 19, 425–436 (2003).
Niu, G. et al. Gene therapy with dominant-negative STAT3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 59, 5059–5063 (1999). The first validation of STAT3 as a target for cancer therapy in an animal model.
Niu, G. et al. Overexpression of a dominant-negative signal transducer and activator of transcription 3 variant in tumor cells leads to production of soluble factors that induce apoptosis and cell cycle arrest. Cancer Res. 61, 3276–3280 (2001).
Takeda, K. et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl Acad. Sci. USA 94, 3801–3804 (1997).
Akira, S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene 19, 2607–2611 (2000).
Liu, X. et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186 (1997).
Socolovsky, M., Fallon, A. E., Wang, S., Brugnara, C. & Lodish, H. F. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-XL induction. Cell 98, 181–191 (1999).
Teglund, S. et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850 (1998).
Battle, T. E. & Frank, D. A. The role of STATs in apoptosis. Curr. Mol. Med. 2, 381–392 (2002).
Turkson, J. et al. Phosphotyrosyl peptides block Stat3-mediated DNA-binding activity, gene regulation and cell transformation. J. Biol. Chem. 276, 45443–45455 (2001). Proof of principle that small peptide molecules can disrupt STAT3 dimerization and block cell transformation.
Blaskovich, M. A. et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 63, 1270–1279 (2003).
Konnikova, L., Kotecki, M., Kruger, M. M. & Cochran, B. H. Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer 3, 23 (2003).
Leong, P. et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc. Natl Acad. Sci. USA 100, 4138–4143 (2003).
Starr, R. & Hilton, D. J. Negative regulation of the JAK/STAT pathway. Bioessays 21, 47–52 (1999).
Naka, T., Fujimoto, M. & Kishimoto, T. Negative regulation of cytokine signaling: STAT-induced STAT inhibitor. Trends Biochem. Sci. 24, 394–398 (1999).
Shuai, K. Modulation of STAT signaling by STAT-interacting proteins. Oncogene 19, 2638–2644 (2000).
Galm, O., Yoshikawa, H., Esteller, M., Osieka, R. & Herman, J. G. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood 101, 2784–2788 (2003).
Yoshikawa, H. et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nature Genet. 28, 29–35 (2001).
Chen, C. Y. et al. SOCS1 methylation in patients with newly diagnosed acute myeloid leukemia. Genes Chromosomes Cancer 37, 300–305 (2003).
Zhang, Q. et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J. Immunol. 168, 466–474 (2002).
Turkson, J. et al. Novel peptidomimetic inhibitors of Stat3 dimerization and biological activity. Mol. Cancer Ther. (in the press).
Berg, T. et al. Small-molecular antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl Acad. Sci. USA 99, 3830–3835 (2002). First example of a small-molecule inhibitor of transcription-factor dimerization that blocks cell transformation.
Acknowledgements
We thank members of our laboratories for stimulating discussions, A. Levitzki for inspiring the title of this review, R. Buettner for assistance with references, J. Brugger for first drafts of the figures, and A. Bruce for secretarial assistance. Work in the authors' laboratories was supported by grants from the NIH, the Dr. Tsai-Fan Yu Endowment for Cancer Research and the Angela Musette Russo Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Glossary
- DANGER SIGNALS
-
Inflammatory mediators produced during viral or bacterial infection that alert the immune system to danger. These signals include cytokines, chemokines and other physiological mediators, such as nitric oxide.
- T-CELL TOLERANCE
-
The inability of T cells to respond to danger signals. It is induced when T cells are initially exposed to antigens in the absence of danger signals. Tolerance results in the suppression of immune responses to tumours.
- BYSTANDER EFFECT
-
The indirect inhibition or killing of tumour cells that are adjacent to those directly affected by gene therapy or pharmacological inhibitors. This could involve soluble factors that are released by apoptotic cancer cells or immune responses.
- DECOY OLIGONUCLEOTIDE
-
A short stretch of synthetic DNA that contains the cognate DNA-binding site of a transcription factor and thereby serves to sequester and functionally inactivate that factor.
- PEPTIDOMIMETICS
-
Small, organic molecules that mimic short stretches of amino acids and can be engineered to bind competitively to native proteins, and can therefore be used as drugs that disrupt protein function.
Rights and permissions
About this article
Cite this article
Yu, H., Jove, R. The STATs of cancer — new molecular targets come of age. Nat Rev Cancer 4, 97–105 (2004). https://doi.org/10.1038/nrc1275
Issue Date:
DOI: https://doi.org/10.1038/nrc1275
This article is cited by
-
5-Methoxytryptophan enhances the sensitivity of sorafenib on the inhibition of proliferation and metastasis for lung cancer cells
BMC Cancer (2024)
-
Interplay of miRNAs and lncRNAs in STAT3 signaling pathway in colorectal cancer progression
Cancer Cell International (2024)
-
STAT3 gene polymorphisms and susceptibility to breast cancer in the Moroccan population
Egyptian Journal of Medical Human Genetics (2023)
-
Elevated LILRB1 expression predicts poor prognosis and is associated with tumor immune infiltration in patients with glioma
BMC Cancer (2023)
-
STAT proteins in cancer: orchestration of metabolism
Nature Reviews Cancer (2023)