Key Points
-
Chemokines are a subset of cytokines that cause the directed migration of leukocytes along a chemical gradient of ligand, known as the chemokine gradient. More than 50 human chemokines and 18 chemokine receptors have been discovered so far.
-
Many cancers have a complex chemokine network that influences the immune-cell infiltration of a tumour, as well as tumour cell growth, survival and migration, and angiogenesis.
-
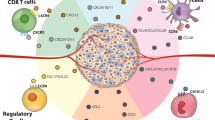
Immune cells, endothelial cells and tumour cells themselves express chemokine receptors and can respond to chemokine gradients.
-
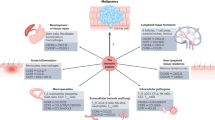
Chemokines are a key determinant of the macrophage and lymphocyte infiltrate of human cancers and might contribute to T-helper 2 cell polarization.
-
Malignant cells from different cancer types have different profiles of chemokine-receptor expression, but CXCR4 is most commonly found; at the last count, cells from 23 different cancer types expressed this receptor.
-
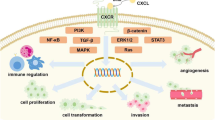
Mutations in genes that alter levels of hypoxia-inducible factor, or gene-fusion events, can induce CXCR4 in cells that do not normally express this receptor. CXCR4 is also transiently increased by factors such as hypoxia, vascular endothelial growth factor and oestrogen in the tumour microenvironment.
-
Studies of human cancer biopsy sampler and mouse cancer models show that cancer cell chemokine-receptor expression is associated with increased metastatic capacity.
-
Preliminary laboratory data show that chemokine-receptor antagonists inhibit macrophage infiltrates, can induce tumour growth arrest or apoptosis, and prevent metastatic spread.
-
Research into the cancer chemokine network is revealing parallels between the pathology of inflammation and malignancy, parallels that enhance our understanding of both types of disease and indicate new approaches for treatment.
Abstract
A complex network of chemokines and their receptors influences the development of primary tumours and metastases. New information about the biological role of chemokines in these processes is providing insights into host–tumour interactions, such as the role of the leukocyte infiltrate, and into the mechanisms that determine the metastatic potential and site-specific spread of cancer cells. Chemokine-receptor antagonists are showing promise in animal models of inflammation and autoimmune disease. Could manipulating the local chemokine network have therapeutic benefits in malignant disease?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rossi, D. & Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 (2000).
Murphy, P. M. et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52, 145–176 (2000). An encyclopedic review of the chemokine universe.
Zlotnik, A. & Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000).
Locati, M. et al. Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J. Immunol. 168, 3557–3562 (2002).
Gupta, S. K., Lysko, P. G., Pillarisetti, K., Ohlstein, E. & Stadel, J. M. Chemokine receptors in human endothelial cells. J. Biol. Chem. 273, 4282–4287 (1998).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001). Experiments on the involvement of chemokines and their receptors in metastasis.
Scotton, C. J., Wilson, J. L., Milliken, D., Stamp, G. & Balkwill, F. R. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 61, 4961–4965 (2001).
Balkwill, F. The significance of cancer cell expression of CXCR4. Semin. Cancer Biol. 14, 171–179 (2004).
Balkwill, F. Chemokine biology in cancer. Semin. Immunol. 15, 49–55 (2003).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow. Lancet 357, 539–545 (2001).
Brigati, C., Noonan, D. M., Albini, A. & Benelli, R. Tumors and inflammatory infiltrates: friends or foes? Clin. Exp. Metastasis 19, 247–258 (2002).
Coussens, L. M. & Werb, Z. Cancer and inflammation. Nature 420, 860–867 (2002).
Negus, R. P. M., Stamp, G. W. H., Hadley, J. & Balkwill, F. R. A quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am. J. Pathol. 150, 1723–1734 (1997).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nature Rev. Cancer 4, 71–78 (2004).
Bottazzi, B. et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science 220, 210–212 (1983).
Negus, R. P. M. et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J. Clin. Invest. 95, 2391–2396 (1995).
Milliken, D., Scotton, C., Raju, S., Balkwill, F. & Wilson, J. Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin. Cancer Res. 8, 1108–1114 (2002).
Skinnider, B. F. & Mak, T. W. The role of cytokines in classical Hodgkin lymphoma. Blood 99, 4283–4297 (2002).
Scotton, C., Milliken, D., Wilson, J., Raju, S. & Balkwill, F. Analysis of CC chemokine and chemokine receptor expression in solid ovarian tumours. Br. J. Cancer 85, 891–897 (2001).
Sica, A. et al. Defective expression of the monocyte chemotactic protein-1 receptor CCR2 in macrophages associated with human ovarian carcinoma. J. Immunol. 164, 733–738 (2000).
Grimshaw, M. J. & Balkwill, F. R. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation: a potential mechanism. Eur. J. Immunol. 31, 480–489 (2001).
Sozzani, S. et al. The viral chemokine macrophage inflammatory protein-II is a selective TH2 chemoattractant. Blood 92, 4036–4039 (1998).
Sica, A. et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-κB activation in tumor-associated macrophages. J. Immunol. 164, 762–767 (2000).
Gu, L. et al. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404, 407–411 (2000).
Zou, W. et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature Med. 7, 1339–1346 (2001).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002). Excellent review on macrophages and tumour progression.
Robinson, S. C., Scott, K. A. & Balkwill, F. R. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-α. Eur. J. Immunol. 32, 404–412 (2002).
Bingle, L., Brown, N. J. & Lewis, C. E. The role of tumour associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 196, 254–265 (2002).
Mantovani, A., Bottazzi, B., Colotta, F., Sozzani, S. & Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 13, 265–270 (1992).
Lin, E. Y., Nguyen, A. V., Russell, R. G. & Pollard, J. W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–739 (2001). Interesting paper indicating that tumour-associated macrophages might aid metastatic spread.
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Daniel, D. et al. Immune enhancement of skin carcinogenesis by CD4+ T cells. J. Exp. Med. 197, 1017–1028 (2003).
Azenshtein, E. et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 62, 1093–1102 (2002).
Saji, H. et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92, 1085–1091 (2001).
Luboshits, G. et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 59, 4681–4687 (1999).
Ohta, M. et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int. J. Cancer 102, 220–224 (2002).
Nesbit, M., Schaider, H., Miller, T. H. & Herlyn, M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumour formation in nontumorigenic melanoma cells. J. Immunol. 166, 6483–6490 (2001).
Monti, P. et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 63, 7451–7461 (2003).
Moran, C. J. et al. RANTES expression is a predictor of survival in stage I lung adenocarcinoma. Clin. Cancer Res. 8, 3803–3812 (2002).
Struyf, S. et al. PARC/CCL18 is a plasma CC chemokine with increased levels in childhood acute lymphoblastic leukemia. Am. J. Pathol. 163, 2065–2075 (2003).
Murphy, P. M. Chemokines and molecular basis of cancer metastasis. N. Engl. J. Med. 354, 833–835 (2001).
Allavena, P. et al. The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol. Rev. 177, 141–149 (2000).
Kijima, T. et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 62, 6304–6311 (2002).
Corcione, A. et al. Stromal cell-derived factor-1 as a chemoattractant for follicular center lymphoma B cells. J. Natl Cancer Inst. 92, 628–635 (2000).
Zhou, Y., Larsen, P. H., Hao, C. & Yong, V. W. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J. Biol. Chem. 277, 49481–49487 (2002).
Koshiba, T. et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin. Cancer Res. 6, 3530–3535 (2000).
Scotton, C. J. et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 62, 5930–5938 (2002).
Hwang, J. H. et al. CXC chemokine receptor 4 expression and function in human anaplastic thyroid cancer cells. J. Clin. Endocrinol. Metab. 88, 408–416 (2003).
Geminder, H. et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases and neuroblastoma. J. Immunol. 167, 4747–4757 (2001).
Tachibana, K. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 (1998).
Zou, Y. -R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Lapidot, T. & Kollet, O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2mnull mice. Leukemia 16, 1992–2003 (2002).
Ara, T. et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J. Immunol. 170, 4649–4655 (2003).
Egawa, T. et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity 15, 323–334 (2001).
Sun, Y. -X. et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell Biochem. 89, 462–473 (2003). Comprehensive study of CXCR4 expression in one cancer type.
Jordan, N. J. et al. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J. Clin. Invest. 104, 1061–1069 (1999).
Bachelder, R. E., Wendt, M. A. & Mercurio, A. M. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 62, 7203–7206 (2002).
Hall, J. M. & Korach, K. S. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol. Endocrinol. 17, 792–803 (2003).
Helbig, G. et al. NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 278, 21631–21638 (2003).
Staller, P. et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425, 307–311 (2003). Fascinating observation that VHL mutations upregulate CXCR4 — a genetic explanation for chemokine-receptor expression by tumour cells.
Schioppa, T. et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 198, 1391–1402 (2003). This paper complements reference 60. It provides an epigenetic explanation for CXCR4 expression on cancer cells.
Libura, J. et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood 100, 2597–2606 (2002).
Kato, M., Kitayama, J., Kazama, S. & Nagawa, H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 5, 144–150 (2003).
Darash-Yahana, M. et al. Human prostate cancer growth, vascularisation and metastasis is stimulated via high expression levels of the chemokine receptor CXCR4. Proc. Am. Assoc. Cancer Res. 44, 789 (2003).
Murakami, T. et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 62, 7328–7334 (2002).
Cardones, A. R., Murakami, T. & Hwang, S. T. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in viva via β-integrin. Cancer Res. 63, 6751–6757 (2003).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Phillips, R. J. et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am. J. Respir. Crit. Care Med. 167, 1676–1686 (2003).
Barbero, S. et al. Stromal cell-derived factor 1a stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 63, 1969–1974 (2003).
Zeelenberg, I. S., Ruuls-Van Stalle, L. & Roos, E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 63, 3833–3839 (2003). Shows that cancer cells in which CXCR4 has been downregulated still spread to the lungs but they do not proliferate to form metastatic deposits.
Moore, R. et al. Tumour necrosis factor-α deficient mice are resistant to skin carcinogenesis. Nature Med. 5, 828–831 (1999).
Szlosarek, P. & Balkwill, F. Tumour necrosis factor-α: a potential target in the therapy of solid tumors. Lancet Oncol. 4, 565–573 (2003).
Dick, J. E. & Lapidot, T. Stem cells take a shortcut to the bone marrow. Blood 101, 2901–2902 (2003).
Petit, I. et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunol. 3, 687–694 (2002).
Levesque, J. -P., Hendy, J., Takamatsu, Y., Simmons, P. J. & Bendall, L. J. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J. Clin. Invest. 111, 187–196 (2003).
Kollet, O. et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 112, 160–169 (2003).
De Clercq, E. The bicyclam AMD3100 story. Nature Rev. Drug Discov. 2, 581–587 (2003).
Liles, W. C. et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 102, 2728–2730 (2003).
Schrader, A. J. et al. CXCR4/CXCL12 expression and signalling in kidney cancer. Br. J. Cancer 86, 1250–1256 (2002).
Mashino, K. et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 62, 2937–2941 (2002).
Takanami, I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int. J. Cancer 105, 186–189 (2003).
Ding, Y. et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin. Cancer Res. 9, 3406–3412 (2003).
Till, K. J., Lin, K., Zuzel, M. & Cawley, J. C. The chemokine receptor CCR7 and α4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood 99, 2977–2984 (2002).
Kleinhans, M. et al. Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T-cell lymphoma. Blood 101, 1487–1493 (2003).
Ishida, T. et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 9, 3625–3634 (2003).
Kleeff, J. et al. Detection and localization of MIP-3α/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int. J. Cancer 81, 650–657 (1999).
Manes, S. et al. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J. Exp. Med. 198, 1381–1389 (2003).
Dhawan, P. & Richmond, A. Role of CXCL1 in tumorigenesis of melanoma. J. Leukoc. Biol. 72, 9–18 (2002).
Yang, T. Y. et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191, 445–453 (2000).
Murakami, T. et al. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J. Exp. Med. 198, 1337–1347 (2003).
Wiley, H. E., Gonzalez, E. B., Maki, S., Wu, M. -T. & Hwang, S. T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl Cancer Inst. 93, 1638–1643 (2001).
Proudfoot, A. E. I. Chemokine receptors: multifaceted therapeutic targets. Nature Rev. Immunol. 2, 106–115 (2002).
Belperio, J. A. et al. CXC chemokines in angiogenesis. J. Leukoc. Biol. 68, 1–8 (2000).
Proudfoot, A. E. et al. Amino-terminally modified RANTES analogues demonstrate differential effects on RANTES receptors. J. Biol. Chem. 274, 32478–32485 (1999).
Robinson, S. C. et al. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 63, 8360–8365 (2003). Proof-of-principle study showing that chemokine-receptor antagonists inhibit the macrophage infiltrate in experimental tumours and slow down tumour growth.
Zeelenberg, I. S., Ruuls-Van Stalle, L. & Roos, E. Retention of CXCR4 in the endoplasmic reticulum blocks dissemination of a cell hybridoma. J. Clin. Invest. 108, 269–277 (2001).
Bertolini, F. et al. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res. 62, 3106–3112 (2002).
Rubin, J. B. et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc. Natl Acad. Sci. USA 100, 13513–13518 (2003). Translational study showing that a chemokine-receptor antagonist acts directly on tumour cells to inhibit tumour growth in the brain.
Signore, A., Capriotti, G., Scopinaro, F., Bonanno, E. & Modesti, A. Radiolabelled lymphokines and growth factors for in vivo imaging of inflammation, infection and cancer. Trends Immunol. 24, 395–402 (2003).
Homey, B., Muller, A. & Zlotnik, A. Chemokines: agents for the immunotherapy of cancer? Nature Rev. Immunol. 2, 175–184 (2002).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Acknowledgements
The author wishes to thank J. Wilson, H. Kulbe and V. Slettenaar for stimulating discussion.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Fran Balkwill is a consultant for Centocor Inc. — the manufacturer of Infliximab (an anti-TNF antibody), which is used in the treatment of inflammatory disease.
Related links
Related links
DATABASES
Cancer.gov
Entrez Gene
Glossary
- CHEMOKINE
-
This term refers to a family of chemotactic cytokines. These small, inducible cytokines are leukocyte-subtype-selective chemoattractants. Some chemokines also have other cytokine-like actions on cells including stimulation of cell proliferation.
- HYPOXIA
-
This term describes the low levels of oxygen that are found in many areas of advanced cancers and are associated with a poor prognosis.
- ASCITES
-
An increased accumulation of fluid in the peritoneum owing to cancer, which both increases the accumulation of peritoneal fluid and inhibits its reabsorption. In some cancers, such as ovarian cancer, the ascitic fluid contains variable numbers of tumour cells and inflammatory leuokytes, as well as pico- to nanomolar levels of many cytokines and chemokines.
- CD8+ T LYMPHOCYTE
-
A T cell bearing the CD8+ cell-surface glycoprotein, which recognizes major histocompatibility complex class I molecules on target cells. CD8+ T cells are usually cytotoxic T cells.
- REED–STERNBERG CELLS
-
A clonal population of transformed germinal-centre B cells that forms the malignant component in Hodgkin's lymphoma.
- TH2 CELLS
-
T-helper 2 (TH2) lymphocytes help B cells make antibody and suppress the action of cytotoxic T cells. Interleukin (IL)-4, IL-5, IL-10 and IL-13 are examples of TH2-produced cytokines.
- TH1 CELLS
-
T-helper 1 (TH1) lymphocytes are at the other end of the functional spectrum to TH2 lymphocytes. They activate macrophages, produce TH1 cytokines — such as interferon-γ and interleukin 2 — and help in the production of specific cytotoxic T lymphocytes.
- TYPE-2 MACROPHAGES
-
Also known as M2 macrophages. Type-2 macrophages have an important role in inflammatory circuits that promote tumour growth and progression. They are polarized cells that are induced by TH2 cytokines and make immunosuppressive cytokines, such as interleukin-10.
- ANTIGEN-PRESENTING CELLS
-
(APCs). Cells that posses antigen and present antigen fragments to lymphocytes in order to initiate an immune response. Dendritic cells are the most potent APCs.
- MATRIX METALLOPROTEINASES
-
A family of proteolytic enzymes that degrade the extracellular matrix and have important roles in tissue remodelling and tumour metastasis.
- PROLIFERATIVE INDEX
-
Describes the numbers of cells that are proliferating in a tissue. Measured by staining cells with antibodies such as Ki67.
- AUTOCRINE
-
A form of bioregulation in which a secretory factor affects the cell from which it was secreted.
- RHABDOMYOSARCOMA
-
A highly lethal mesenchymal cancer of early childhood, which is most commonly found in the eye, head and neck region, and the genitourinary tract.
- SCID
-
Severe combined immunodeficiency. Mice with this defect do not make T cell or antibody responses. Tumour cells from another species can be grown in these mice without rejection.
- LEUKOCYTOSIS
-
The state of increased numbers of leukocytes circulating in the peripheral blood.
Rights and permissions
About this article
Cite this article
Balkwill, F. Cancer and the chemokine network. Nat Rev Cancer 4, 540–550 (2004). https://doi.org/10.1038/nrc1388
Issue Date:
DOI: https://doi.org/10.1038/nrc1388
This article is cited by
-
Automated synthesis and quality control of [68Ga]Ga-PentixaFor using the Gaia/Luna Elysia-Raytest module for CXCR4 PET imaging
EJNMMI Radiopharmacy and Chemistry (2023)
-
The synergistic and enhancive effects of IL-6 and M-CSF to expand and differentiate functional dendritic cells from human monocytes under serum-free condition
Journal of Biological Engineering (2023)
-
Dissemination feature based on PET/CT is a risk factor for diffuse large B cell lymphoma patients outcome
BMC Cancer (2023)
-
CXCL5 and CXCL14, but not CXCL16 as potential biomarkers of colorectal cancer
Scientific Reports (2023)
-
Anti-tumor effects of anti-programmed cell death-1 antibody treatment are attenuated in streptozotocin-induced diabetic mice
Scientific Reports (2023)