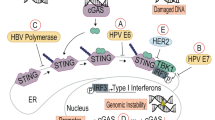
Abstract
The ability to fuse cells is shared by many viruses, including common human pathogens and several endogenous viruses. Here we will discuss how cell fusion can link viruses to cancer, what types of cancers it can affect, how the existence of this link can be tested and how the hypotheses that we propose might affect the search for human oncogenic viruses. In particular, we will focus on the ability of cell fusion that is caused by viruses to induce chromosomal instability, a common affliction of cancer cells that has been thought to underlie the malignant properties of cancerous tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jemal, A. et al. Cancer statistics, 2007. CA Cancer J. Clin. 57, 43–66 (2007).
Doll, R. & Boreham, J. Recent trends in cancer mortality in the UK. Br. J. Cancer 92, 1329–1335 (2005).
Susser, M. & Stein, Z. Civilisation and peptic ulcer. Lancet 1, 115–119 (1962).
Kidd, M. & Modlin, I. M. A century of Helicobacter pylori: paradigms lost—paradigms regained. Digestion 59, 1–15 (1998).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
zur Hausen, H. Infections Causing Human Cancer (Wiley-VCH, Weinheim, 2006).
Roden, R. & Wu, T. C. How will HPV vaccines affect cervical cancer? Nature Rev. Cancer 6, 753–763 (2006).
Chien, Y. C., Jan, C. F., Kuo, H. S. & Chen, C. J. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol. Rev. 28, 126–135 (2006).
Beasley, R. P. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 61, 1942–1956 (1988).
zur Hausen, H. Viruses in human tumors—personal reflections. Behring Inst. Mitt. 91, 21–27 (1992).
Evans, A. S. & Mueller, N. E. Viruses and cancer. Causal associations. Ann. Epidemiol. 1, 71–92 (1990).
Duelli, D. M., Hearn, S., Myers, M. P. & Lazebnik, Y. A primate virus generates transformed human cells by fusion. J. Cell Biol. 171, 493–503 (2005).
Parris, G. E. The role of viruses in cell fusion and its importance to evolution, invasion and metastasis of cancer clones. Med. Hypotheses 64, 1011–1014 (2005).
Marsh, M. & Helenius, A. Virus entry: open sesame. Cell 124, 729–740 (2006).
Dimitrov, D. S. Virus entry: molecular mechanisms and biomedical applications. Nature Rev. Microbiol. 2, 109–122 (2004).
Chen, E. H. & Olson, E. N. Unveiling the mechanisms of cell–cell fusion. Science 308, 369–373 (2005).
Shmulevitz, M. & Duncan, R. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 19, 902–912 (2000).
Liu, T. C. & Kirn, D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 67, 429–432 (2007).
Ogle, B. M., Cascalho, M. & Platt, J. L. Biological implications of cell fusion. Nature Rev. Mol. Cell Biol. 6, 567–575 (2005).
Chen, E. H., Grote, E., Mohler, W. & Vignery, A. Cell–cell fusion. FEBS Lett. 581, 2181–2193 (2007).
Duelli, D. & Lazebnik, Y. Cell fusion: a hidden enemy? Cancer Cell 3, 445–448 (2003).
Ringertz, N. R. & Savage, R. E. Cell Hybrids (Academic Press, New York, 1976).
Ogle, B. M. et al. Spontaneous fusion of cells between species yields transdifferentiation and retroviral transfer in vivo. FASEB J. 18, 548–550 (2004).
Larizza, L. & Schirrmacher, V. Somatic cell fusion as a source of genetic rearrangement leading to metastatic variants. Cancer Metastasis Rev. 3, 193–222 (1984).
Ganem, N. J., Storchova, Z. & Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. (2007).
Shackney, S. E. et al. Model for the genetic evolution of human solid tumors. Cancer Res. 49, 3344–3354 (1989).
Margolis, R. L. Tetraploidy and tumor development. Cancer Cell 8, 353–354 (2005).
Boveri, T. The Origin of Malignant Tumors (The Williams & Wilkins Company, Baltimore, 1929).
Duesberg, P., Li, R., Fabarius, A. & Hehlmann, R. The chromosomal basis of cancer. Cell Oncol. 27, 293–318 (2005).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Storchova, Z. & Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nature Rev. Mol. Cell Biol. 5, 45–54 (2004).
Steinbeck, R. G., Heselmeyer, K. M. & Auer, G. U. DNA ploidy in human colorectal adenomas. Anal. Quant. Cytol. Histol 16, 196–202 (1994).
Kronenwett, U., Huwendiek, S., Castro, J., Ried, T. & Auer, G. Characterisation of breast fine-needle aspiration biopsies by centrosome aberrations and genomic instability. Br. J. Cancer 92, 389–395 (2005).
Yu, C., Zhang, X., Huang, Q., Klein, M. & Goyal, R. K. High-fidelity DNA histograms in neoplastic progression in Barrett's esophagus. Lab. Invest. 87, 466–472 (2007).
Matzke, M. A., Mette, M. F., Kanno, T. & Matzke, A. J. Does the intrinsic instability of aneuploid genomes have a causal role in cancer? Trends Genet. 19, 253–256 (2003).
FitzPatrick, D. R. Transcriptional consequences of autosomal trisomy: primary gene dosage with complex downstream effects. Trends Genet. 21, 249–253 (2005).
Munzarova, M. & Kovarik, J. Is cancer a macrophage-mediated autoaggressive disease? Lancet 1, 952–954 (1987).
Vignery, A. Macrophage fusion: are somatic and cancer cells possible partners? Trends Cell Biol. 15, 188–193 (2005).
Pawelek, J. M. Tumour cell hybridization and metastasis revisited. Melanoma Res. 10, 507–514 (2000).
Lagarde, A. E. & Kerbel, R. S. Somatic cell hybridization in vivo and in vitro in relation to the metastatic phenotype. Biochim. Biophys. Acta 823, 81–110 (1985).
Jacobsen, B. M. et al. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 66, 8274–8279 (2006).
Rodic, N., Rutenberg, M. S. & Terada, N. Cell fusion and reprogramming: resolving our transdifferences. Trends Mol. Med. 10, 93–96 (2004).
Tada, T. Nuclear reprogramming: an overview. Methods Mol. Biol. 348, 227–236 (2006).
Bjerkvig, R., Tysnes, B. B., Aboody, K. S., Najbauer, J. & Terzis, A. J. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nature Rev. Cancer 5, 899–904 (2005).
Houghton, J. et al. Gastric cancer originating from bone marrow-derived cells. Science 306, 1568–1571 (2004).
Marx, J. Medicine. Bone marrow cells: the source of gastric cancer? Science 306, 1455–1457 (2004).
Andersen, T. et al. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: a new type of cancer-host partnership? J. Pathol. 211, 10–17 (2007).
Wiener, F., Fenyo, E. M. & Klein, G. Tumor-host cell hybrids in radiochimeras. Proc. Natl Acad. Sci. USA 71, 148–152 (1974).
Mortensen, K., Lichtenberg, J., Thomsen, P. D. & Larsson, L. I. Spontaneous fusion between cancer cells and endothelial cells. Cell. Mol. Life Sci. 61, 2125–2131 (2004).
Goldenberg, D. M., Pavia, R. A. & Tsao, M. C. In vivo hybridisation of human tumour and normal hamster cells. Nature 250, 649–651. (1974).
Kikyo, N. & Wolffe, A. P. Reprogramming nuclei: insights from cloning, nuclear transfer and heterokaryons. J. Cell Sci. 113, 11–20. (2000).
Cowan, C. A., Atienza, J., Melton, D. A. & Eggan, K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 (2005).
Zhang, F., Pomerantz, J. H., Sen, G., Palermo, A. T. & Blau, H. M. Active tissue-specific DNA demethylation conferred by somatic cell nuclei in stable heterokaryons. Proc. Natl Acad. Sci. USA 104, 4395–4400 (2007).
Sullivan, S. & Eggan, K. The potential of cell fusion for human therapy. Stem Cell Rev. 2, 341–349 (2006).
Bergsmedh, A. et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl Acad. Sci. USA 98, 6407–6411 (2001).
Bergsmedh, A., Szeles, A., Spetz, A. L. & Holmgren, L. Loss of the p21(Cip1/Waf1) cyclin kinase inhibitor results in propagation of horizontally transferred DNA. Cancer Res. 62, 575–579 (2002).
Duelli, D. M. et al. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr. Biol. 17, 431–437 (2007).
Heselmeyer, K. et al. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc. Natl Acad. Sci. USA 93, 479–484 (1996).
Heselmeyer, K. et al. Advanced-stage cervical carcinomas are defined by a recurrent pattern of chromosomal aberrations revealing high genetic instability and a consistent gain of chromosome arm 3q. Genes Chromosomes Cancer 19, 233–240 (1997).
Kronenwett, U. et al. Improved grading of breast adenocarcinomas based on genomic instability. Cancer Res. 64, 904–909 (2004).
Steinbeck, R. G., Heselmeyer, K. M., Neugebauer, W. F., Falkmer, U. G. & Auer, G. U. DNA ploidy in human colorectal adenocarcinomas. Anal. Quant. Cytol. Histol 15, 187–194 (1993).
Weger, A. R. et al. Nuclear DNA distribution pattern of the parenchymal cells in adenocarcinomas of the pancreas and in chronic pancreatitis. A study of archival specimens using both image and flow cytometry. Gastroenterology 99, 237–242 (1990).
Takeuchi, M. et al. Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7, and hTERT genes. In Vitro Cell Dev. Biol. Anim. 43, 129–138 (2007).
Duensing, S. & Munger, K. Centrosomes, genomic instability, and cervical carcinogenesis. Crit. Rev. Eukaryot. Gene Expr. 13, 9–23 (2003).
Tysnes, B. B. & Bjerkvig, R. Cancer initiation and progression: involvement of stem cells and the microenvironment. Biochim. Biophys. Acta 1775, 283–297 (2007).
White, J. & Dalton, S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 1, 131–138 (2005).
Alvarez-Dolado, M. Cell fusion: biological perspectives and potential for regenerative medicine. Front. Biosci. 12, 1–12 (2007).
Wang, X. et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 (2003).
Elgui de Oliveira, D. DNA viruses in human cancer: an integrated overview on fundamental mechanisms of viral carcinogenesis. Cancer Lett. 247, 182–196 (2007).
Pagano, J. S. et al. Infectious agents and cancer: criteria for a causal relation. Semin. Cancer Biol. 14, 453–471 (2004).
Mant, C., Hodgson, S., Hobday, R., D'Arrigo, C. & Cason, J. A viral aetiology for breast cancer: time to re-examine the postulate. Intervirology 47, 2–13 (2004).
Wiernik, P. H. & Etkind, P. R. Is mouse mammary tumor virus an etiologic agent of human breast cancer and lymphoma? South. Med. J. 99, 108–110 (2006).
Rakowicz-Szulczynska, E. M., McIntosh, D. G. & Smith, M. L. Giant syncytia and virus-like particles in ovarian carcinoma cells isolated from ascites fluid. Clin. Diagn Lab. Immunol. 6, 115–126 (1999).
Johnson, R. T. & Rao, P. N. Mammalian cell fusion: induction of premature chromosome condensation in interphase nuclei. Nature 226, 717–722 (1970).
Kovacs, G. Premature chromosome condensation: evidence for in vivo cell fusion in human malignant tumours. Int. J. Cancer 36, 637–641 (1985).
Williams, D. M., Scott, C. D. & Beck, T. M. Premature chromosome condensation in human leukemia. Blood 47, 687–693 (1976).
Petkovic, I. et al. Premature chromosome condensation in children with acute lymphocytic leukemia (L1) and malignant histiocytosis. Cancer Genet. Cytogenet. 35, 37–40 (1988).
Miles, C. P. & Wolinska, W. A comparative analysis of chromosomes and diagnostic cytology in effusions from 58 cancer patients. Cancer 32, 1458–1469 (1973).
Reichmann, A. & Levin, B. Premature chromosome condensation in human large bowel cancer. Cancer Genet. Cytogenet. 3, 221–225 (1981).
Atkin, N. B. Premature chromosome condensation in carcinoma of the bladder: presumptive evidence for fusion of normal and malignant cells. Cytogenet. Cell Genet. 23, 217–219 (1979).
Sreekantaiah, C., Bhargava, M. K. & Shetty, N. J. Premature chromosome condensation in human cervical carcinoma. Cancer Genet. Cytogenet. 24, 263–269 (1987).
Casalone, R., Meriggi, F., Forni, E. & Pasquali, F. Cytogenetic findings in a case of anaplastic carcinoma of the pancreas. Cancer Genet. Cytogenet. 29, 253–259 (1987).
Sandberg, A. A. Some comments regarding chromosome pulverization (premature chromosome condensation or PCC, prophasing). Virchows Arch. B Cell Pathol. 29, 15–18 (1978).
Deckard-Janatpour, K. et al. Tumors of the pancreas with osteoclast-like and pleomorphic giant cells: an immunohistochemical and ploidy study. Arch. Pathol. Lab. Med. 122, 266–272 (1998).
Sakai, Y. et al. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol. 31, 1223–1229 (2000).
Moyes, D., Griffiths, D. J. & Venables, P. J. Insertional polymorphisms: a new lease of life for endogenous retroviruses in human disease. Trends Genet. 23, 326–333 (2007).
Dewannieux, M., Blaise, S. & Heidmann, T. Identification of a functional envelope protein from the HERV-K family of human endogenous retroviruses. J. Virol. 79, 15573–15577 (2005).
Kleiman, A. et al. HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in patients with germ cell tumors. Int. J. Cancer 110, 459–461 (2004).
Herbst, H., Sauter, M. & Mueller-Lantzsch, N. Expression of human endogenous retrovirus K elements in germ cell and trophoblastic tumors. Am. J. Pathol. 149, 1727–1735 (1996).
Atkin, N. B. & Baker, M. C. High chromosome numbers of testicular germ cell tumors. An update. Cancer Genet. Cytogenet. 84, 90 (1995).
Buscher, K. et al. Expression of the human endogenous retrovirus-K transmembrane envelope, Rec. and Np9 proteins in melanomas and melanoma cell lines. Melanoma Res. 16, 223–234 (2006).
Buscher, K. et al. Expression of human endogenous retrovirus K in melanomas and melanoma cell lines. Cancer Res. 65, 4172–4180 (2005).
Thompson, F. H. et al. Cytogenetics of 158 patients with regional or disseminated melanoma. Subset analysis of near-diploid and simple karyotypes. Cancer Genet. Cytogenet. 83, 93–104 (1995).
Nelson, M. A. et al. Chromosome abnormalities in malignant melanoma: clinical significance of nonrandom chromosome abnormalities in 206 cases. Cancer Genet. Cytogenet. 122, 101–109 (2000).
Wang-Johanning, F. et al. Expression of human endogenous retrovirus k envelope transcripts in human breast cancer. Clin. Cancer Res. 7, 1553–1560 (2001).
Wang-Johanning, F. et al. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer 120, 81–90 (2007).
Wang-Johanning, F. et al. Detecting the expression of human endogenous retrovirus E envelope transcripts in human prostate adenocarcinoma. Cancer 98, 187–197 (2003).
Blaise, S., de Parseval, N. & Heidmann, T. Functional characterization of two newly identified human endogenous retrovirus coding envelope genes. Retrovirology 2, 19 (2005).
Blond, J. L. et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74, 3321–3329 (2000).
Bjerregaard, B., Holck, S., Christensen, I. J. & Larsson, L. I. Syncytin is involved in breast cancer-endothelial cell fusions. Cell. Mol. Life Sci. 63, 1906–1911 (2006).
Strick, R. et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-β. J. Mol. Med. 85, 23–38 (2007).
Pradhan, M., Abeler, V. M., Danielsen, H. E., Trope, C. G. & Risberg, B. A. Image cytometry DNA ploidy correlates with histological subtypes in endometrial carcinomas. Mod. Pathol. 19, 1227–1235 (2006).
Going, J. J. Epithelial carcinogenesis: challenging monoclonality. J. Pathol. 200, 1–3 (2003).
Levy, A. Monoclonality of endocrine tumours: What does it mean? Trends Endocrinol. Metab. 12, 301–307 (2001).
Pageau, G. J., Hall, L. L., Ganesan, S., Livingston, D. M. & Lawrence, J. B. The disappearing Barr body in breast and ovarian cancers. Nature Rev. Cancer 7, 628–633 (2007).
Katona, T. M. et al. Genetically heterogeneous and clonally unrelated metastases may arise in patients with cutaneous melanoma. Am. J. Surg. Pathol. 31, 1029–1037 (2007).
Pawelek, J. M. Tumour-cell fusion as a source of myeloid traits in cancer. Lancet Oncol. 6, 988–993 (2005).
Duelli, D. M. & Lazebnik, Y. A. Primary cells suppress oncogene-dependent apoptosis. Nature Cell Biol. 2, 859–862 (2000).
Harris, H. How tumour suppressor genes were discovered. FASEB J. 7, 978–979 (1993).
Miller, F. R., Mohamed, A. N. & McEachern, D. Production of a more aggressive tumor cell variant by spontaneous fusion of two mouse tumor subpopulations. Cancer Res. 49, 4316–4321 (1989).
Ying, Q. L., Nichols, J., Evans, E. P. & Smith, A. G. Changing potency by spontaneous fusion. Nature 416, 545–548 (2002).
Anisimov, V. N., Ukraintseva, S. V. & Yashin, A. I. Cancer in rodents: does it tell us about cancer in humans? Nature Rev. Cancer 5, 807–819 (2005).
Aractingi, S. et al. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 65, 1755–1760 (2005).
Peters, B. A. et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nature Med. 11, 261–262 (2005).
Cambier, J. L. & Wheeless, L. L. The binucleate cell: implications for automated cytopathology. Acta Cytol. 19, 281–285 (1975).
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005).
Jefford, C. E. & Irminger-Finger, I. Mechanisms of chromosome instability in cancers. Crit. Rev. Oncol. Hematol. 59, 1–14 (2006).
Weaver, B. A. & Cleveland, D. W. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 18, 658–667 (2006).
Kops, G. J., Weaver, B. A. & Cleveland, D. W. On the road to cancer: aneuploidy and the mitotic checkpoint. Nature Rev. Cancer 5, 773–785 (2005).
Rajagopalan, H. & Lengauer, C. Aneuploidy and cancer. Nature 432, 338–341 (2004).
Bailey, S. M. & Murnane, J. P. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 34, 2408–2417 (2006).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649. (1998).
Storchova, Z. et al. Genome-wide genetic analysis of polyploidy in yeast. Nature 443, 541–547 (2006).
Otto, S. P. & Whitton, J. Polyploid incidence and evolution. Annu. Rev. Genet. 34, 401–437 (2000).
Gallardo, M. H., Bickham, J. W., Honeycutt, R. L., Ojeda, R. A. & Kohler, N. Discovery of tetraploidy in a mammal. Nature 401, 341 (1999).
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007).
Rao, P. N. & Johnson, R. T. Premature chromosome condensation: a mechanism for the elimination of chromosomes in virus-fused cells. J. Cell Sci. 10, 495–513 (1972).
Matsui, S. “Prophasing” as a possible cause of chromosome translocation in virus-fused cells. Nature New Biol. 243, 208–209 (1973).
Walter, M. A. & Goodfellow, P. N. Radiation hybrids: irradiation and fusion gene transfer. Trends Genet. 9, 352–356 (1993).
Gupta, S. Hepatic polyploidy and liver growth control. Semin. Cancer Biol. 10, 161–171 (2000).
Maohuai, C., Chang, A. R. & Lo, D. Nasopharyngeal carcinoma heterogeneity of DNA content identified on cytologic preparations. Anal. Quant. Cytol. Histol. 23, 213–217 (2001).
Baba, H. et al. DNA ploidy and its clinical implications in gastric cancer. Surgery 131, S63–S70 (2002).
Acknowledgements
We thank our collaborators for their commitment and for discussions and we thank the Sondock family for financial support and for encouragement.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Glossary
- Aneuploidy
-
Any deviation from the exact multiple of the euploid number of chromosomes for the species. This includes a deviation in the number of whole chromosomes (numerical aneuploidy) and in parts of the chromosomes (segmental aneuploidy).
- Cell hybrids
-
Mononuclear cells produced by mitosis of heterokaryons. The best-known example of cell hybrids are hybridomas.
- Chromosomal instability
-
(CIN). An abnormally high frequency of chromosomal aberrations in a cell or cell population, such as chromosome losses, gains or translocations. Chromosomal instability leads to aneuploidy.
- Heterokaryon
-
Multinuclear cells produced by fusion of different cells.
- Osteoclasts
-
Syncytia whose function is to dissolve bone.
- Syncytium
-
A cell produced by fusion that has more than a few nuclei.
Rights and permissions
About this article
Cite this article
Duelli, D., Lazebnik, Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer 7, 968–976 (2007). https://doi.org/10.1038/nrc2272
Issue Date:
DOI: https://doi.org/10.1038/nrc2272
This article is cited by
-
Cell fusion enhances energy metabolism of mesenchymal tumor hybrid cells to sustain their proliferation and invasion
BMC Cancer (2021)
-
Stoichiometric analysis of protein complexes by cell fusion and single molecule imaging
Scientific Reports (2020)
-
Polyploidy in liver development, homeostasis and disease
Nature Reviews Gastroenterology & Hepatology (2020)
-
Fusion-mediated chromosomal instability promotes aneuploidy patterns that resemble human tumors
Oncogene (2019)
-
Histone stress: an unexplored source of chromosomal instability in cancer?
Current Genetics (2019)