Key Points
-
Nicotinic acetylcholine receptors (nAChRs) are ion channels that are expressed in the plasma membrane of all mammalian cells, including cancer cells.
-
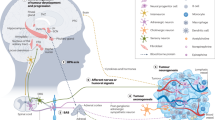
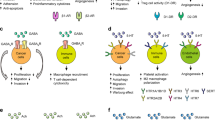
nAChRs are central regulators that stimulate the synthesis and release of stimulatory and inhibitory neurotransmitters, which in turn regulate the release of growth, angiogenic and neurogenic factors and stimulate signal transduction in a cell-type-specific manner.
-
Chronic exposure to nicotine or nicotine-derived carcinogenic nitrosamines upregulates cancer-stimulatory nAChRs and desensitizes cancer-inhibitory nAChRs.
-
The homomeric α7nAChR is the major stimulator of cancer development and progression: it induces the synthesis and release of autocrine growth factors in small-cell lung cancer and indirectly stimulates most other types of cancer through the systemic and cellular release of stress neurotransmitters that bind as agonists to β-adrenergic receptors.
-
The heteromeric α4β2nAChR is a major inhibitor of cancer development and progression: it stimulates the release of γ-aminobutyric acid, which blocks the cancer stimulating effects of β-adrenergic receptors by inhibiting cyclic AMP.
-
Marker-guided cancer intervention strategies that target nAChRs and their effectors that aim to restore the balance between stimulatory and inhibitory neurotransmitters need to be developed.
Abstract
Nicotinic acetylcholine receptors (nAChRs) are the central regulators of stimulatory and inhibitory neurotransmitters that control the synthesis and release of growth, angiogenic and neurotrophic factors in cancer cells, the cancer microenvironment and distant organs. Data discussed in this Review suggests that smoking and possibly other environmental and lifestyle factors increase the function of nAChRs that stimulate cancer cells and reduce the function of nAChRs that inhibit cancer cells. This novel paradigm necessitates the development of marker-guided cancer intervention strategies that aim to restore the balance between nAChR-mediated stimulatory and inhibitory neurotransmitters and their downstream effectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Burns, D. M. Tobacco-related diseases. Semin. Oncol. Nurs. 19, 244–249 (2003).
Brambilla, E., Travis, W. D., Colby, T. V., Corrin, B. & Shimosato, Y. The new World Health Organization classification of lung tumours. Eur. Respir. J. 18, 1059–1068 (2001).
Khuder, S. A. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 31, 139–148 (2001).
Hecht, S. S. Tobacco smoke carcinogens and lung cancer. J. Natl Cancer Inst. 91, 1194–1210 (1999).
Benowitz, N. L. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. 46, 91–111 (2003).
Benowitz, N. L. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am. J. Med. 121, S3–S10 (2008).
Gotti, C., Fornasari, D. & Clementi, F. Human neuronal nicotinic receptors. Prog. Neurobiol. 53, 199–237 (1997).
Schuller, H. M. Cell type specific, receptor-mediated modulation of growth kinetics in human lung cancer cell lines by nicotine and tobacco-related nitrosamines. Biochem. Pharmacol. 38, 3439–3442 (1989). This was the first report that implicated nAChRs in the growth regulation of cancer.
Maneckjee, R. & Minna, J. D. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc. Natl Acad. Sci. USA 87, 3294–3298 (1990). This was the first report that implicated nAChRs in the regulation of cancer cell apoptosis.
Schuller, H. M. & Orloff, M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem. Pharmacol. 55, 1377–1384 (1998). This study identified cancer-causing nitrosamines as agonists for nAChRs.
Wessler, I. & Kirkpatrick, C. J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 54, 1558–1571 (2008). This review details the ubiquitous expression of nAChRs in all cells and tissues.
Sobel, A., Weber, M. & Changeux, J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur. J. Biochem. 80, 215–224 (1977).
O'Brian, R. D., Eldefrawi, A. T. & Eldefrawi, A. T. Isolation of acetylcholine receptors. Annu. Rev. Pharmacol. 12, 19–34 (1972).
Karlin, A. The acetylcholine receptor: progress report. Life Sci. 14, 1385–1415 (1974).
Galzi, J. L., Revah, F., Bessis, A. & Changeux, J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu. Rev. Pharmacol. Toxicol. 31, 37–72 (1991).
Portugal, G. S. & Gould, T. J. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav. Brain Res. 193, 1–16 (2008).
Lindstrom, J. et al. Structure and function of neuronal nicotinic acetylcholine receptors. Prog. Brain Res. 109, 125–137 (1996).
Le Novere, N. & Changeux, J. P. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J. Mol. Evol. 40, 155–172 (1995).
Gopalakrishnan, M. et al. Stable expression and pharmacological properties of the human alpha 7 nicotinic acetylcholine receptor. Eur. J. Pharmacol. 290, 237–246 (1995).
Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 205, 159–173 (2005).
Roderick, H. L. & Cook, S. J. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nature Rev. Cancer 8, 361–375 (2008).
Kawai, H. & Berg, D. K. Nicotinic acetylcholine receptors containing the α7 subunits on rat cortical neurons do not undergo long lasting inactivation even when upregulated by chronic nicotine exposure. J. Neurochem. 78, 1367–1378 (2001). This study showed that, unlike heteromeric nAChRs, α7nAChR is not desensitized by chronic nicotine.
Barik, J. & Wonnacott, S. Indirect modulation by α7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol. Pharmacol. 69, 618–628 (2006).
Lang, K. et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer 112, 231–238 (2004).
Schuller, H. M., Al-Wadei, H. A. & Majidi, M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis (2008).
Schuller, H. M., Al-Wadei, H. A. & Majidi, M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer 112, 767–778 (2008).
Drell, T. L.t. et al. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat. 80, 63–70 (2003).
Joseph, J., Niggemann, B., Zaenker, K. S. & Entschladen, F. The neurotransmitter γ-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 62, 6467–6469 (2002). This was the first report that implicated GABA as a tumour suppressor.
Nakazawa, K. & Ohno, Y. Block by phytoestrogens of recombinant human neuronal nicotinic receptors. J. Pharmacol. Sci. 93, 118–121 (2003).
Devesa, S. S., Bray, F., Vizcaino, A. P. & Parkin, D. M. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer 117, 294–299 (2005).
Takahashi, H. K. et al. The immunosuppressive effects of nicotine during mixed lymphocyte reaction. Eur. J. Pharmacol. 559, 69–74 (2007).
Kawashima, K., Yoshikawa, K., Fujii, Y. X., Moriwaki, Y. & Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 80, 2314–2319 (2007).
Cattaneo, M. G., Codignola, A., Vicentini, L. M., Clementi, F. & Sher, E. Nicotine stimulates a serotonergic autocrine loop in human small-cell lung carcinoma. Cancer Res. 53, 5566–5568 (1993). This study showed for the first time that nAChRs stimulate the growth of SCLC by releasing the autocrine growth factor serotonin.
Jull, B. A., Plummer, H. K. 3rd & Schuller, H. M. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J. Cancer Res. Clin. Oncol. 127, 707–717 (2001).
Gil-Ad, I. et al. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int. J. Oncol. 33, 277–286 (2008).
Nocito, A. et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 68, 5152–5158 (2008).
Olincy, A., Leonard, S., Young, D. A., Sullivan, B. & Freedman, R. Decreased bombesin peptide response to cigarette smoking in schizophrenia. Neuropsychopharmacology 20, 52–59 (1999).
Haass, M. & Kubler, W. Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther. 19, 657–665 (1997).
Mozayan, M. & Lee, T. J. Statins prevent cholinesterase inhibitor blockade of sympathetic α7nAChR-mediated currents in rat superior cervical ganglion neurons. Am. J. Physiol. Heart Circ. Physiol. 293, H1737–H1744 (2007).
Wong, H. P. et al. Nicotine promotes cell proliferation via α7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol. Appl. Pharmacol. 221, 261–267 (2007). This report showed for the first time that cancer cells synthesize and release stress neurotransmitters in response to α7nAChR stimulation.
Verhoeckx, K. C., Doornbos, R. P., Witkamp, R. F., van der Greef, J. & Rodenburg, R. J. Beta-adrenergic receptor agonists induce the release of granulocyte chemotactic protein-2, oncostatin M, and vascular endothelial growth factor from macrophages. Int. Immunopharmacol. 6, 1–7 (2006).
Grau, M., Soley, M. & Ramirez, I. Interaction between adrenaline and epidermal growth factor in the control of liver glycogenolysis in mouse. Endocrinology 138, 2601–2609 (1997).
Schuller, H. M., Tithof, P. K., Williams, M. & Plummer, H. 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a β-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via β-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 59, 4510–4515 (1999).
Weddle, D. L., Tithoff, P., Williams, M. & Schuller, H. M. β-Adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis 22, 473–479 (2001).
Masur, K., Niggemann, B., Zanker, K. S. & Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 61, 2866–2869 (2001).
Palm, D. et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. Int. J. Cancer 118, 2744–2749 (2006).
Shin, V. Y. et al. Functional role of β-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol. Sci. 96, 21–29 (2007).
Thaker, P. H. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Med. 12, 939–944 (2006).
Plummer, H. K. 3rd, Dhar, M. S., Cekanova, M. & Schuller, H. M. Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer 5, 104 (2005).
Arredondo, J., Chernyavsky, A. I. & Grando, S. A. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol. Ther. 5, 511–517 (2006).
Sheppard, B. J., Williams, M., Plummer, H. K. & Schuller, H. M. Activation of voltage-operated Ca2+-channels in human small cell lung carcinoma by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int. J. Oncol. 16, 513–518 (2000).
Plummer, H. K. 3rd, Sheppard, B. J. & Schuller, H. M. Interaction of tobacco-specific toxicants with nicotinic cholinergic regulation of fetal pulmonary neuroendocrine cells: implications for pediatric lung disease. Exp. Lung Res. 26, 121–135 (2000).
Plummer, H. K. 3rd, Dhar, M. & Schuller, H. M. Expression of the α7 nicotinic acetylcholine receptor in human lung cells. Respir. Res. 6, 29 (2005).
Codignola, A. et al. Serotonin release and cell proliferation are under the control of a-bungarotoxin-sensitive nicotinic receptors in small-cell lung carcinoma cell lines. FEBS Lett. 342, 286–290 (1994).
Sartelet, H. et al. Expression of nicotinic receptors in normal and tumoral pulmonary neuroendocrine cells (PNECDs). Pathol. Res. Pract. 204, 891–898 (2008).
Xu, L. & Deng, X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induces phosphorylation of μ- and m-calpain in association with increased secretion, cell migration, and invasion. J. Biol. Chem. 279, 53683–53690 (2004).
Jin, Z., Gao, F., Flagg, T. & Deng, X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J. Biol. Chem. 279, 40209–40219 (2004).
Seufferlein, T. & Rozengurt, E. Galanin, neurotensin, and phorbol esters rapidly stimulate activation of mitogen-activated protein kinase in small cell lung cancer cells. Cancer Res. 56, 5758–5764 (1996).
Schuller, H. M., Orloff, M. & Reznik, G. K. Inhibition of protein-kinase-C-dependent cell proliferation of human lung cancer cell lines by the dihydropyridine dexniguldipine. J. Cancer Res. Clin. Oncol. 120, 354–358 (1994).
Shafer, S. H., Phelps, S. H. & Williams, C. L. Reduced DNA synthesis and cell viability in small cell lung carcinoma by treatment with cyclic AMP phosphodiesterase inhibitors. Biochem. Pharmacol. 56, 1229–1236 (1998).
Pursiheimo, J. P., Kieksi, A., Jalkanen, M. & Salmivirta, M. Protein kinase A balances the growth factor-induced Ras/ERK signaling. FEBS Lett. 521, 157–164 (2002).
Heeschen, C., Weis, M., Aicher, A., Dimmeler, S. & Cooke, J. P. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J. Clin. Invest. 110, 527–536 (2002). This was the first report that identified the α7nAChR as a regulator of angiogenesis.
Grozio, A. et al. Nicotine, lung and cancer. Anticancer Agents Med. Chem. 7, 461–466 (2007).
Cooke, J. P. Angiogenesis and the role of the endothelial nicotinic acetylcholine receptor. Life Sci. 80, 2347–2351 (2007).
Schuller, H. M. Carbon dioxide potentiates the mitogenic effects of nicotine and its carcinogenic derivative, NNK, in normal and neoplastic neuroendocrine lung cells via stimulation of autocrine and protein kinase C-dependent mitogenic pathways. Neurotoxicology 15, 877–886 (1994).
Schuller, H. M. Nitrosamines as nicotinic receptor ligands. Life Sci. 80, 2274–2280 (2007).
Schuller, H. M. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs 19, 655–671 (2008).
Purdue, M. P. et al. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 62, 51–56 (2007).
Mannino, D. M. Chronic obstructive pulmonary disease: definition and epidemiology. Respir. Care 48, 1185–1191; discussion 1191–1193 (2003).
Gwilt, C. R., Donnelly, L. E. & Rogers, D. F. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol. Ther. 115, 208–222 (2007).
Schuller, H. M., Porter, B. & Riechert, A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J. Cancer Res. Clin. Oncol. 126, 624–630 (2000).
Boswell-Smith, V. & Spina, D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int. J. Chron. Obstruct. Pulmon. Dis. 2, 121–129 (2007).
Wang, D. & Cui, X. Evaluation of PDE4 inhibition for COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 1, 373–379 (2006).
Schuller, H. M., McGavin, M. D., Orloff, M., Riechert, A. & Porter, B. Simultaneous exposure to nicotine and hyperoxia causes tumors in hamsters. Lab. Invest. 73, 448–456 (1995). This study showed that nicotine causes neuroendocrine lung cancer in hamsters with COPD but was non-carcinogenic in healthy hamsters.
Schuller, H. M. et al. Pathobiology of lung tumors induced in hamsters by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the modulating effect of hyperoxia. Cancer Res. 50, 1960–1965 (1990).
Schuller, H. M., Porter, B., Riechert, A., Walker, K. & Schmoyer, R. Neuroendocrine lung carcinogenesis in hamsters is inhibited by green tea or theophylline while the development of adenocarcinomas is promoted: implications for chemoprevention in smokers. Lung Cancer 45, 11–18 (2004).
Egleton, R. D., Brown, K. C. & Dasgupta, P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol. Sci. 29, 151–158 (2008).
West, K. A. et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 111, 81–90 (2003). This study provided first evidence that the frequent overexpression of Akt in NSCLC is caused by nAChR stimulation.
Tsurutani, J. et al. Tobacco components stimulate Akt-dependent proliferation and NFκB-dependent survival in lung cancer cells. Carcinogenesis 26, 1182–1195 (2005).
Laag, E. et al. NNK activates ERK1/2 and CREB/ATF-1 via β-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int. J. Cancer 119, 1547–1552 (2006).
Majidi, M., Al-Wadei, H. A., Takahashi, T. & Schuller, H. M. Nongenomic β estrogen receptors enhance β1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cells. Cancer Res. 67, 6863–6871 (2007).
Brognard, J., Clark, A. S., Ni, Y. & Dennis, P. A. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 61, 3986–3997 (2001).
Dasgupta, P. et al. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc. Natl Acad. Sci. USA 103, 6332–6337 (2006).
Dasgupta, P. et al. Nicotine induces cell proliferation by β-arrestin-mediated activation of Src and Rb–Raf-1 pathways. J. Clin. Invest. 116, 2208–2217 (2006).
Arredondo, J., Chernyavsky, A. I. & Grando, S. A. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J. Cancer Res. Clin. Oncol. 132, 653–663 (2006).
Martinez-Garcia, E., Irigoyen, M., Anso, E., Martinez-Irujo, J. J. & Rouzaut, A. Recurrent exposure to nicotine differentiates human bronchial epithelial cells via epidermal growth factor receptor activation. Toxicol. Appl. Pharmacol. 228, 334–342 (2008).
Epperson, C. N. et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol. Psychiatry 57, 44–48 (2005). This study showed reduced GABA in the brain of smokers.
Lam, D. C. et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 67, 4638–4647 (2007). This was the first report that associated the downregulation of α4nAChRs with lung adenocarcinoma.
Shin, V. Y. et al. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis 25, 2487–2495 (2004).
Wong, H. P. et al. Nicotine promotes colon tumor growth and angiogenesis through β-adrenergic activatriton. Toxicol. Sci. 97, 279–287 (2007).
Askari, M. D., Tsao, M. S. & Schuller, H. M. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through β-adrenergic transactivation of EGF receptors. J. Cancer Res. Clin. Oncol. 131, 639–648 (2005).
Arredondo, J., Chernyavsky, A. I. & Grando, S. A. SLURP-1 and -2 in normal, immortalized and malignant oral keratinocytes. Life Sci. 80, 2243–2247 (2007). This study provided evidence that SLURP1 may be useful for cancer intervention by desensitizing nAChRs.
Arredondo, J., Chernyavsky, A. I., Jolkovsky, D. L., Pinkerton, K. E. & Grando, S. A. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of α7 nicotinic receptor in oral keratinocytes. FASEB J. 20, 2093–2101 (2006).
Arredondo, J., Chernyavsky, A. I., Jolkovsky, D. L., Pinkerton, K. E. & Grando, S. A. Receptor-mediated tobacco toxicity: alterations of the NF-κB expression and activity downstream of α7 nicotinic receptor in oral keratinocytes. Life Sci. 80, 2191–2194 (2007).
Arredondo, J., Chernyavsky, A. I., Jolkovsky, D. L., Webber, R. J. & Grando, S. A. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J. Cell Physiol. 208, 238–245 (2006).
Arredondo, J., Chernyavsky, A. I., Jolkovsky, D. L., Pinkerton, K. E. & Grando, S. A. Receptor-mediated tobacco toxicity: acceleration of sequential expression of alpha5 and alpha7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J. 22, 1356–1368 (2008).
Liu, X. et al. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J. Cell Biochem. 105, 53–60 (2008).
Liu, X. et al. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of β-adrenoceptors. J. Pharmacol. Exp. Ther. 326, 69–75 (2008).
Trombino, S. et al. α7-Nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: role of mitogen-activated protein kinase pathway. Cancer Res. 64, 135–145 (2004).
Chen, R., Ho, Y., Guo, H. & Wang, Y. Rapid activation of Stat3 and ERK1/2 by nicotine modulates cell proliferarion in human bladder cancer cells. Toxicol. Sci. 104, 283–293 (2008).
Cheodotal, A., Kerjan, G. & Moreau-Fauvarque, C. The brain within the tumor: new roled for axon guidance molecules in cancers. Cell Death Differ. 12, 1044–1056 (2005). This study provided first evidence suggesting that the growth of cancer is supported by the formation of new nerve endings (neurogenesis).
Palm, D. & Entschladen, F. in Neuronal activity in tumor tissue (eds Zänker, K. S. & Entschladen, F.) 91–98 (Karger, Basel, 2007).
Entschladen, F., Palm, D., Niggemann, B. & Zaenker, K. S. The cancer's nervous tooth: Considering the neuronal crosstalk within tumors. Semin. Cancer Biol. 18, 171–175 (2008).
Zafra, F., Lindholm, D., Castren, E., Hartikka, J. & Thoenen, H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J. Neurosci. 12, 4793–4799 (1992).
French, S. J., Humby, T., Horner, C. H., Sofroniew, M. V. & Rattray, M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res. Mol. Brain Res. 67, 124–136 (1999).
Girault, J. A. & Greengard, P. The neurobiology of dopamine signaling. Arch. Neurol. 61, 641–644 (2004).
Cakir, Y., Plummer, H. K., 3rd, Tithof, P. K. & Schuller, H. M. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int. J. Oncol. 21, 153–157 (2002).
Sood, A. K. et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 12, 369–375 (2006).
Yang, E. V. et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 66, 10357–10364 (2006).
Thorgeirsson, T. E. et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642 (2008).
Shiraishi, K. et al. Contribution of nicotine acetylcholine receptor polymorphisms to lung cancer risk in a smoking-independent manner in the Japanese. Carcinogenesis 12 Nov 2008 (doi:10.1093/carcin/bgn257).
Schuller, H. M. Nitrosamines as nitotinic receptor ligands. Life Sci. 80, 2274–2280 (2007).
Abnet, C. C. Carcinogenic food contaminants. Cancer Invest. 25, 189–196 (2007).
Jakszyn, P. & Gonzales, C. A. Nitrosamine and related food intake and gastric and esophafeal cancer risk: a systematic review of the epidemiological evidence. World J. Gastroenterol. 12, 4296–4303 (2006).
Sato, M. et al. Multiple oncogenic changes (K-RasV12, p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 66, 2116–2128 (2006).
Leonard, S. et al. Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry 59, 1085–1096 (2002).
Al-Wadei, H. A. N., Takahashi, T. & Schuller, H. M. Theophylline stimulates cAMP-mediated signaling associated with growth regulation in human cells from pulmonary adenocarcinoma and small airway epithelia. Int. J. Oncol. 27, 27–155 (2005).
Al-Wadei, H. A., Takahashi, T. & Schuller, H. M. Caffeine stimulates the proliferation of human lung adenocarcinoma cells and small airway epithelial cells via activation of, PKA, CREB and ERK1/2. Oncol. Rep. 15, 431–435 (2006).
Al-Wadei, H. A., Takahashi, T. & Schuller, H. M. Growth stimulation of human pulmonary adenocarcinoma cells and small airway epithelial cells by beta-carotene via activation of cAMP, PKA, CREB and ERK1/2. Int. J. Cancer 118, 1370–1380 (2006).
Aggarwal, S. et al. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol. Biol. Cell 17, 566–575 (2006).
Al-Wadei, H. A. & Schuller, H. M. Cyclic adenosine monophosphate-dependent cell type-specific modulation of mitogenic signaling by retinoids in normal and neoplastic lung cells. Cancer Detect Prev. 30, 403–411 (2006).
Pereira, G. E. et al. Microclimate influence on mineral and metabolic profiles of grape berries. J. Agric. Food Chem. 54, 6765–6775 (2006).
Bianchini, F. & Vainio, H. Wine and resveratrol: mechanisms of cancer prevention? Eur. J. Cancer Prev. 12, 417–425 (2003).
Schoonen, W. M., Salinas, C. A., Kiemeney, L. A. & Stanford, J. L. Alcohol consumption and risk of prostate cancer in middle-aged men. J. Urol. 173, 1170 (2005).
Author information
Authors and Affiliations
Glossary
- Nicotine-derived nitrosamines
-
Cancer-causing agents formed from nicotine by nitrosation.
- Ion channel
-
A channel in the plasma membrane through which ions flow from the outside to the interior of the cell.
- Desensitization
-
An nAChR is desensitized by a reversible conformational change in the receptor that is characterized by a significant decrease in responsiveness to an agonist.
- Phyto-oestrogens
-
A naturally occurring compound derived from plants or plant products that acts like oestrogen in the body.
- β-Adrenergic receptor
-
G-protein-coupled receptors in effector tissues, most of which are innervated by adrenergic post-ganglionic fibres of the sympathetic nervous system and are activated by noradrenaline, adrenaline and various adrenergic drugs.
- Calpains
-
Proteolytic enzymes that are regulated by Ca2+.
- Chronic obstructive pulmonary disease
-
(COPD). A chronic inflammation of the lungs.
Rights and permissions
About this article
Cite this article
Schuller, H. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors?. Nat Rev Cancer 9, 195–205 (2009). https://doi.org/10.1038/nrc2590
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2590
This article is cited by
-
The tobacco-specific carcinogen NNK induces pulmonary tumorigenesis via nAChR/Src/STAT3-mediated activation of the renin-angiotensin system and IGF-1R signaling
Experimental & Molecular Medicine (2023)
-
Tumor-specific cholinergic CD4+ T lymphocytes guide immunosurveillance of hepatocellular carcinoma
Nature Cancer (2023)
-
Targeting ADRB2 enhances sensitivity of non-small cell lung cancer to VEGFR2 tyrosine kinase inhibitors
Cell Death Discovery (2022)
-
Neurotransmitter signaling: a new frontier in colorectal cancer biology and treatment
Oncogene (2022)
-
Nerve growth factor interacts with CHRM4 and promotes neuroendocrine differentiation of prostate cancer and castration resistance
Communications Biology (2021)