Key Points
-
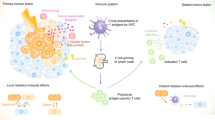
Radiation-induced bystander responses are defined as the response of cells to their neighbours being irradiated. These have been observed in a range of cell types and measured for a range of end points.
-
Long-range, abscopal (out-of-field) effects have also been observed after the clinical use of radiation.
-
The main mechanisms involve direct cell–cell communication by gap junction intercellular communication and release of factors into the medium.
-
Bystander signalling has a key role in increasing the effectiveness of gene therapy approaches in which common mechanisms involving cytokine signalling and the production of reactive oxygen and nitrogen species have been used to maximize effectiveness.
-
With the development of suitable strategies, radiation-induced bystander responses may be used to enhance tumour cell kill or protect normal tissues from the damaging consequences of radiation exposure.
Abstract
Our understanding of how radiation kills normal and tumour cells has been based on an intimate knowledge of the direct induction of DNA damage and its cellular consequences. What has become clear is that, as well as responses to direct DNA damage, cell–cell signalling — known as the bystander effect — mediated through gap junctions and inflammatory responses may have an important role in the response of cells and tissues to radiation exposure and also chemotherapy agents. This Review outlines the key aspects of radiation-induced intercellular signalling and assesses its relevance for existing and future radiation-based therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hall, E. J. & Giaccia, A. J. Radiobiology for the Radiologist (Lippincott William & Wilkins, Philadelphia, 2006).
Harper, J. W. & Elledge, S. J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007).
Jackson, S. P. Detecting, signalling and repairing double-strand breaks. Biochem. Soc. Trans. 29, 655–661 (2001).
Morgan, W. F. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 159, 567–580 (2003). Good starting point for a review of cellular data on radiation-induced bystander responses.
Morgan, W. F. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat. Res. 159, 581–596 (2003).
Hamada, N., Matsumoto, H., Hara, T. & Kobayashi, Y. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J. Radiat. Res. (Tokyo) 48, 87–95 (2007).
Mothersill, C. & Seymour, C. B. Radiation-induced bystander effects — implications for cancer. Nature Rev. Cancer 4, 158–164 (2004).
Bagdonas, S., Dahle, J., Kaalhus, O. & Moan, J. Cooperative inactivation of cells in microcolonies treated with UVA radiation. Radiat. Res. 152, 174–179 (1999).
Dahle, J. et al. The bystander effect in photodynamic inactivation of cells. Biochim. Biophys. Acta 1475, 273–280 (2000).
DeVeaux, L. C., Durtschi, L. S., Case, J. G. & Wells, D. P. Bystander effects in unicellular organisms. Mutat. Res. 597, 78–86 (2006).
Alexandre, J., Hu, Y., Lu, W., Pelicano, H. & Huang, P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 67, 3512–3517 (2007).
Ramesh, R., Marrogi, A. J., Munshi, A., Abboud, C. N. & Freeman, S. M. In vivo analysis of the 'bystander effect': a cytokine cascade. Exp. Hematol. 24, 829–838 (1996).
Mesnil, M., Piccoli, C., Tiraby, G., Willecke, K. & Yamasaki, H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc. Natl Acad. Sci. USA 93, 1831–1835 (1996).
McMasters, R. A. et al. Lack of bystander killing in herpes simplex virus thymidine kinase-transduced colon cell lines due to deficient connexin43 gap junction formation. Hum. Gene Ther. 9, 2253–2261 (1998).
Denning, C. & Pitts, J. D. Bystander effects of different enzyme–prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum. Gene Ther. 8, 1825–1835 (1997).
Kaminski, J. M. et al. The controversial abscopal effect. Cancer Treat. Rev. 31, 159–172 (2005). A good review of clinical data supporting long-range or abscopal radiation-induced bystander responses.
Nagasawa, H. & Little, J. B. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 52, 6394–6396 (1992). A defining paper of radiation-induced bystander responses in cellular models.
Belyakov, O. V., Malcolmson, A. M., Folkard, M., Prise, K. M. & Michael, B. D. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br. J. Cancer 84, 674–679 (2001).
Schettino, G., Folkard, M., Michael, B. D. & Prise, K. M. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused CK X rays. Radiat. Res. 163, 332–336 (2005).
Shareef, M. M. et al. Role of tumor necrosis factor-α and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 67, 11811–11820 (2007).
Schettino, G. et al. Low-dose studies of bystander cell killing with targeted soft X rays. Radiat. Res. 160, 505–511 (2003).
Kadhim, M. A. et al. Bystander-mediated genomic instability in human lymphocytes after single α-particle irradiation. Radiat. Res. 161, 110–111 (2004).
Prise, K. M., Belyakov, O. V., Folkard, M. & Michael, B. D. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int. J. Radiat. Biol. 74, 793–798 (1998). The first direct evidence of radiation-induced bystander effect using a microbeam to irradiate individual cells.
Gaugler, M. H. et al. Intestinal epithelial cell dysfunction is mediated by an endothelial-specific radiation-induced bystander effect. Radiat. Res. 167, 185–193 (2007).
Shao, C., Furusawa, Y., Aoki, M., Matsumoto, H. & Ando, K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 78, 837–844 (2002).
Coates, P. J., Lorimore, S. A. & Wright, E. G. Damaging and protective cell signalling in the untargeted effects of ionizing radiation. Mutat. Res. 568, 5–20 (2004).
Yang, H., Asaad, N. & Held, K. D. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene 24, 2096–2103 (2005).
Huo, L., Nagasawa, H. & Little, J. B. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiat. Res. 156, 521–525 (2001).
Sawant, S. G., Randers-Pehrson, G., Geard, C. R., Brenner, D. J. & Hall, E. J. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat. Res. 155, 397–401 (2001).
Mothersill, C. & Seymour, C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of irradiated cells. Int. J. Radiat. Biol. 71, 421–427 (1997).
Belyakov, O. V., Folkard, M., Mothersill, C., Prise, K. M. & Michael, B. D. Bystander-induced differentiation: A major response to targeted irradiation of a urothelial explant model. Mutat. Res. 597, 43–49 (2006).
Lyng, F. M., Seymour, C. B. & Mothersill, C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat. Res. 157, 365–370 (2002).
Iyer, R. & Lehnert, B. E. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat. Res. 503, 1–9 (2002).
Herve, J. C., Bourmeyster, N., Sarrouilhe, D. & Duffy, H. S. Gap junctional complexes: from partners to functions. Prog. Biophys. Mol. Biol. 94, 29–65 (2007).
Oyamada, M., Oyamada, Y. & Takamatsu, T. Regulation of connexin expression. Biochim. Biophys. Acta 1719, 6–23 (2005).
Azzam, E. I., de Toledo, S. M., Gooding, T. & Little, J. B. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of α particles. Radiat. Res. 150, 497–504 (1998).
Azzam, E. I., de Toledo, S. M. & Little, J. B. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from α-particle irradiated to nonirradiated cells. Proc. Natl Acad. Sci. USA 98, 473–478 (2001). Direct evidence for a role of GJIC in radiation-induced bystander signalling.
Trosko, J. E. & Ruch, R. J. Cell–cell communication in carcinogenesis. Front. Biosci., D208–D236 (1998).
Emerit, I. Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis. Free Radic. Biol. Med. 16, 99–109 (1994).
Emerit, I., Khan, S. H. & Esterbauer, H. Hydroxynonenal, a component of clastogenic factors? Free Radic. Biol. Med. 10, 371–377 (1991).
Auclair, C., Gouyette, A., Levy, A. & Emerit, I. Clastogenic inosine nucleotide as components of the chromosome breakage factor in scleroderma patients. Arch. Biochem. Biophys. 278, 238–244 (1990).
Emerit, I. et al. Superoxide-mediated clastogenesis and anticlastogenic effects of exogenous superoxide dismutase. Proc. Natl Acad. Sci. USA 93, 12799–12804 (1996).
Murphy, J. B., Liu, J. H. & Sturm, E. Studies on X-ray effects: IX. The action of serum from X-rayed animals on lymphoid cells in vitro. J. Exp. Med. 35, 373–384 (1922).
Hollowell, J. G. & Littlefield, G. Chromosome damage induced by plasma of X-rayed patients: an indirect effect of X-ray. Proc. Soc. Exp. Biol. Med. 129, 240–244 (1968).
Emerit, I. et al. Transferable clastogenic activity in plasma from persons exposed as salvage personnel of the Chernobyl reactor. J. Cancer Res. Clin. Oncol. 120, 558–561 (1994).
Chou, C. H. et al. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin. Cancer Res. 13, 851–857 (2007).
Narayanan, P. K., LaRue, K. E., Goodwin, E. H. & Lehnert, B. E. α particles induce the production of interleukin-8 by human cells. Radiat. Res. 152, 57–63 (1999).
Iyer, R. & Lehnert, B. E. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 60, 1290–1298 (2000).
Zhou, H. et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc. Natl Acad. Sci. USA 102, 14641–14646 (2005).
Lehnert, B. E. & Goodwin, E. H. Extracellular factor(s) following exposure to α-particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 57, 2164–2171 (1997).
Shao, C., Stewart, V., Folkard, M., Michael, B. D. & Prise, K. M. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 63, 8437–8442 (2003).
Coates, P. J., Robinson, J. I., Lorimore, S. A. & Wright, E. G. Ongoing activation of p53 pathway responses is a long-term consequence of radiation exposure in vivo and associates with altered macrophage activities. J. Pathol. 214, 610–616 (2008).
Shao, C., Folkard, M., Michael, B. D. & Prise, K. M. Targeted cytoplasmic irradiation induces bystander responses. Proc. Natl Acad. Sci. USA 101, 13495–13500 (2004).
Wu, L. J. et al. Targeted cytoplasmic irradiation with α particles induces mutations in mammalian cells. Proc. Natl Acad. Sci. USA 96, 4959–4964 (1999).
Nugent, S. M. et al. Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct γ radiation and bystander factors. Radiat. Res. 168, 134–142 (2007).
Tartier, L., Gilchrist, S., Burdak-Rothkamm, S., Folkard, M. & Prise, K. M. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 67, 5872–9 (2007).
Mothersill, C. Development of primary tissue culture techniques for use in radiobiology. Radiat. Res. 150, S121–125 (1998).
Mothersill, C. et al. Individual variation in the production of a 'bystander signal' following irradiation of primary cultures of normal human urothelium. Carcinogenesis 22, 1465–1471 (2001).
Mothersill, C. et al. Genetic factors influencing bystander signaling in murine bladder epithelium after low-dose irradiation in vivo. Radiat. Res. 163, 391–399 (2005).
Belyakov, O. V., Folkard, M., Mothersill, C., Prise, K. M. & Michael, B. D. A proliferation-dependent bystander effect in primary porcine and human urothelial explants in response to targeted irradiation. Br. J. Cancer 88, 767–774 (2003).
Belyakov, O. V. et al. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc. Natl Acad. Sci. USA 102, 14203–14208 (2005).
Sedelnikova, O. A. et al. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 67, 4295–4302 (2007).
Travis, E. L. Organizational response of normal tissues to irradiation. Semin. Radiat. Oncol. 11, 184–196 (2001).
Khan, M. A., Hill, R. P. & Van Dyk, J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int. J. Radiat. Oncol. Biol. Phys. 40, 467–476 (1998).
Khan, M. A., Van Dyk, J., Yeung, I. W. & Hill, R. P. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother. Oncol. 66, 95–102 (2003).
Calveley, V. L., Khan, M. A., Yeung, I. W., Vandyk, J. & Hill, R. P. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int. J. Radiat. Biol. 81, 887–899 (2005).
De Ridder, M. et al. IFN-γ+ CD8+ T lymphocytes: possible link between immune and radiation responses in tumor-relevant hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 71, 647–651 (2008).
Moiseenko, V. V., Battista, J. J., Hill, R. P., Travis, E. L. & Van Dyk, J. In-field and out-of-field effects in partial volume lung irradiation in rodents: possible correlation between early DNA damage and functional endpoints. Int. J. Radiat. Oncol. Biol. Phys. 48, 1539–1548 (2000).
Morgan, G. W. & Breit, S. N. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int. J. Radiat. Oncol. Biol. Phys. 31, 361–369 (1995).
Coates, P. J., Rundle, J. K., Lorimore, S. A. & Wright, E. G. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 68, 450–456 (2008). A paper highlighting the important role of macrophage responses in radiation-induced bystander signalling.
Koturbash, I. et al. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene 25, 4267–4275 (2006).
Koturbash, I. et al. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis 28, 1831–1838 (2007).
Koturbash, I. et al. Radiation-induced bystander effects in vivo are sex specific. Mutat. Res. 642, 28–36 (2008).
Koturbash, I. et al. Sex-specific microRNAome deregulation in the shielded bystander spleen of cranially exposed mice. Cell Cycle 7, 1658–1667 (2008).
Mancuso, M. et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl Acad. Sci. USA 105, 12445–12450 (2008). Studies in vivo showing evidence of long-range bystander responses leading to carcinogenesis.
Camphausen, K. et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 63, 1990–1993 (2003).
Brooks, A. L., Retherford, J. C. & McClellan, R. O. Effect of 239PuO2 particle number and size on the frequency and distribution of chromosome aberrations in the liver of the Chinese hamster. Radiat. Res. 59, 693–709 (1974).
Brooks, A. L. et al. The induction of liver tumors by 239Pu citrate or 239PuO2 particles in the Chinese hamster. Radiat. Res. 96, 135–151 (1983).
Barcellos-Hoff, M. H. & Brooks, A. L. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat. Res. 156, 618–627 (2001).
Wang, Z. et al. Adenoviral gene transfer of the human inducible nitric oxide synthase gene enhances the radiation response of human colorectal cancer associated with alterations in tumor vascularity. Cancer Res. 64, 1386–1395 (2004).
Worthington, J. et al. Use of the radiation-inducible WAF1 promoter to drive iNOS gene therapy as a novel anti-cancer treatment. J. Gene Med. 6, 673–680 (2004).
Boyd, M. et al. An efficient targeted radiotherapy/gene therapy strategy utilising human telomerase promoters and radioastatine and harnessing radiation-mediated bystander effects. J. Gene Med. 6, 937–947 (2004).
Burdak-Rothkamm, S., Short, S. C., Folkard, M., Rothkamm, K. & Prise, K. M. ATR-dependent radiation-induced γH2AX foci in bystander primary human astrocytes and glioma cells. Oncogene 26, 993–1002 (2007).
Little, J. B., Nagasawa, H., Li, G. C. & Chen, D. J. Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations. Radiat. Res. 159, 262–267 (2003).
Burdak-Rothkamm, S., Rothkamm, K. & Prise, K. M. ATM acts downstream of ATR in the DNA damage response signalling of bystander cells. Cancer Res. 68, 7059–7065 (2008).
Mothersill, C. & Seymour, C. B. Bystander and delayed effects after fractionated radiation exposure. Radiat. Res. 158, 626–633 (2002).
Gow, M. D., Seymour, C. B., Byun, S. H. & Mothersill, C. E. Effect of dose rate on the radiation-induced bystander response. Phys. Med. Biol. 53, 119–132 (2008).
Fenwick, J. D., Tome, W. A., Soisson, E. T., Mehta, M. P. & Rock Mackie, T. Tomotherapy and other innovative IMRT delivery systems. Semin. Radiat. Oncol. 16, 199–208 (2006).
Milano, M. T., Constine, L. S. & Okunieff, P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin. Radiat. Oncol. 17, 131–140 (2007).
Veldeman, L. et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 9, 367–375 (2008).
Ling, C. C. et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 47, 551–560 (2000).
Verellen, D. et al. Innovations in image-guided radiotherapy. Nature Rev. Cancer 7, 949–960 (2007).
Suchowerska, N., Ebert, M. A., Zhang, M. & Jackson, M. In vitro response of tumour cells to non-uniform irradiation. Phys. Med. Biol. 50, 3041–3051 (2005).
Claridge Mackonis, E. et al. Cellular response to modulated radiation fields. Phys. Med. Biol. 52, 5469–5482 (2007). A paper discussing the role of bystander responses to modulated beams used in IMRT.
Palm, A. & Johansson, K. A. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol. 46, 462–473 (2007).
Dilmanian, F. A. et al. Interlaced x-ray microplanar beams: a radiosurgery approach with clinical potential. Proc. Natl Acad. Sci. USA 103, 9709–9714 (2006).
Dilmanian, F. A. et al. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: is a bystander effect involved? Exp. Hematol. 35, 69–77 (2007).
Hall, E. J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 65, 1–7 (2006).
Bishayee, A., Hill, H. Z., Stein, D., Rao, D. V. & Howell, R. W. Free radical-initiated and gap junction-mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three-dimensional tissue culture model. Radiat. Res. 155, 1–10 (2000).
Xue, L. Y., Butler, N. J., Makrigiorgos, G. M., Adelstein, S. J. & Kassis, A. I. Bystander effect produced by radiolabeled tumor cells in vivo. Proc. Natl Acad. Sci. USA 99, 13765–13770 (2002).
Boyd, M. et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J. Nucl. Med. 47, 1007–1015 (2006). Comparison of effectiveness of different radionuclides at inducing bystander responses in comparison with external beam therapy.
McCready, V. R. & O'Sullivan, J. M. Future directions for unsealed source radionuclide therapy for bone metastases. Eur. J. Nucl. Med. Mol. Imaging 29, 1271–1275 (2002).
Dearling, J. L. & Pedley, R. B. Technological advances in radioimmunotherapy. Clin. Oncol. (R. Coll. Radiol.) 19, 457–469 (2007).
DeNardo, S. J. & Denardo, G. L. Targeted radionuclide therapy for solid tumors: an overview. Int. J. Radiat. Oncol. Biol. Phys. 66, S89–S95 (2006).
Boyd, M., Cunningham, S. H., Brown, M. M., Mairs, R. J. & Wheldon, T. E. Noradrenaline transporter gene transfer for radiation cell kill by 131I meta-iodobenzylguanidine. Gene Ther. 6, 1147–52 (1999).
Boswell, C. A. & Brechbiel, M. W. Development of radioimmunotherapeutic and diagnostic antibodies: an inside-out view. Nucl. Med. Biol. 34, 757–778 (2007).
Brenner, D. J. et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA 100, 13761–13766 (2003).
Acknowledgements
The authors acknowledge the extensive literature they were not able to cite owing to space limits. They are grateful to Cancer Research UK grant number C1513/A7047, the European Union NOTE project (FI6R 036465) and the US National Institutes of Health (5P01CA095227-02) for funding their work.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Photodynamic therapy
-
A therapeutic approach in which photosensitive chemicals are taken up into tumours and then activated by laser or other light source to produce damaging free radicals
- Ganciclovir
-
A synthetic analogue of 2′-deoxyguanosine that becomes phosphorylated when taken up by cells and is incorporated into DNA by DNA polymerase and leads to chain termination of the replicating DNA strands.
- Clastogenic factors
-
A species that can break chromosomes.
- Sister chromatid exchanges
-
Exchange of chromosomal material between the chromatids of a chromosome.
- Lindane
-
A neurotoxin that can inhibit gap-junctional intercellular communication.
- Reconstruct models
-
In vitro models in which individual cell types can be co-cultured and used to form three-dimensional representations of the original tissue.
- Bilateral pneumonitis
-
Inflammation of lung tissue in both lungs.
- Radionuclide therapy
-
The use of radioisotopes tagged to molecules or proteins for treating cancer
- Dose rate
-
The amount of dose delivered per unit time.
- Intensity-modulated radiation therapy
-
IMRT. An advanced mode of radiotherapy that uses multiple modulated beams in which the intensity is varied to allow maximal conformation of the beam delivery to the tumour in three dimensions.
- Linear accelerator
-
A device for the acceleration of subatomic particles that can produce electron beams for radiotherapy.
- Heavy ion particle therapies
-
The use of accelerated beams of high-atomic-mass elements (for example, carbon) for therapy.
- Microplanar X-ray microbeams
-
Parallel beams of X-rays only a few micrometres across that are used for localized irradiation.
- Auger electron cascades
-
Decay of radioactive isotopes by K-shell electron capture leads to the Auger effect, resulting in the loss of several orbital electrons.
- Linear no-threshold hypothesis
-
A model used for radiation protection that aims to describe the relationship between radiation dose and risk, a linear relationship that has no dose threshold for increased risk.
Rights and permissions
About this article
Cite this article
Prise, K., O'Sullivan, J. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 9, 351–360 (2009). https://doi.org/10.1038/nrc2603
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2603
This article is cited by
-
Quantification of biochemical PSA dynamics after radioligand therapy with [177Lu]Lu-PSMA-I&T using a population pharmacokinetic/pharmacodynamic model
EJNMMI Physics (2024)
-
A novel bystander effect in tamoxifen treatment: PPIB derived from ER+ cells attenuates ER− cells via endoplasmic reticulum stress-induced apoptosis
Cell Death & Disease (2024)
-
Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy
Journal of Nanobiotechnology (2023)
-
Immunological and tumor-intrinsic mechanisms mediate the synergistic growth suppression of experimental glioblastoma by radiotherapy and MET inhibition
Acta Neuropathologica Communications (2023)
-
A comparison of the chemo- and radiotoxicity of thorium and uranium at different enrichment grades
Archives of Toxicology (2023)