Key Points
-
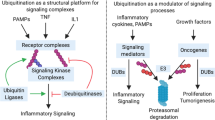
Protracted activation of the immune system by persistent inflammation or chronic infection can be oncogenic.
-
Nuclear factor-κB (NF-κB) transcription factors are normally activated in a transient manner in response to inflammation, infection and other cellular stresses to provide pro-inflammatory and pro-survival signals to regain homeostasis.
-
Failure to downregulate persistent NF-κB signalling can instead facilitate the manifestation of all six hallmarks of cancer: self-sufficient cell growth, insensitivity to growth-inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis.
-
Ubiquitylation is a post-translational modification that tightly regulates the activity of signalling proteins in NF-κB transduction cascades.
-
A20 is a central regulator of inflammation and functions as a ubiquitin-editing enzyme to potently downregulate NF-κB signalling.
-
A20 is inactivated in various haematological malignancies and results in constitutive NF-κB activation in tumour cells; therefore, A20 is a crucial tumour suppressor.
-
The identification of A20 as a tumour suppressor establishes new links between ubiquitylation, NF-κB activation and tumorigenesis that may be exploited in the clinic for improved patient care.
Abstract
Clinicians have suspected for hundreds of years that chronic activation of the immune system contributes to the development of cancer. However, the molecular mechanisms that mediate this precarious interplay are only now being elucidated. Recent reports have identified A20 as a crucial tumour suppressor in various lymphomas. A20 is a ubiquitin-editing enzyme that attenuates the activity of proximal signalling complexes at pro-inflammatory receptors. In this Review we summarize the evidence linking chronic inflammation with tumorigenesis and consider how A20 modulates inflammatory signalling cascades, thereby providing a mechanism to explain how deregulation of ubiquitylation can promote tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McCulloch, C. A., Downey, G. P. & El-Gabalawy, H. Signalling platforms that modulate the inflammatory response: new targets for drug development. Nature Rev. Drug Discov. 5, 864–876 (2006).
Boone, D. L. et al. Recent advances in understanding NF-κB regulation. Inflamm. Bowel Dis. 8, 201–212 (2002).
Karin, M. Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 (2006).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Aggarwal, B. B. & Gehlot, P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr. Opin. Pharmacol. 9, 351–369 (2009).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Karin, M. The IκB kinase — a bridge between inflammation and cancer. Cell Res. 18, 334–342 (2008).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Prasad, S,. Ravindran, J. & Aggarwal, B. B. NF-κB and cancer: how intimate is this relationship. Mol. Cell Biochem. 336, 25–37 (2009).
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006).
Xia, Z. P. & Chen, Z. J. TRAF2: a double-edged sword? Sci. STKE 2005, pe7 (2005).
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004). This work provided definitive evidence in an in vivo system that constitutive NF-κB activity activated by persistent inflammation can promote tumorigenesis.
Becker, C. et al. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Naugler, W. E. & Karin, M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 14, 109–119 (2008).
Luo, J. L., Maeda, S., Hsu, L. C., Yagita, H. & Karin, M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 6, 297–305 (2004).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Baud, V. & Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nature Rev. Drug Discov. 8, 33–40 (2009).
Liu, Y. C., Penninger, J. & Karin, M. Immunity by ubiquitylation: a reversible process of modification. Nature Rev. Immunol. 5, 941–952 (2005).
Dixit, V. M. et al. Tumor necrosis factor-α induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 265, 2973–2978 (1990). The initial characterization of A20 as a cytokine-inducible factor.
Krikos, A., Laherty, C. D. & Dixit, V. M. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J. Biol. Chem. 267, 17971–17976 (1992).
Chen, Z. J. Ubiquitin signalling in the NF-κB pathway. Nature Cell Biol. 7, 758–765 (2005).
Beyaert, R., Heyninck, K. & Van Huffel, S. A20 and A20-binding proteins as cellular inhibitors of nuclear factor-κB-dependent gene expression and apoptosis. Biochem. Pharmacol. 60, 1143–1151 (2000).
Laherty, C. D., Perkins, N. D. & Dixit, V. M. Human T cell leukemia virus type I Tax and phorbol 12-myristate 13-acetate induce expression of the A20 zinc finger protein by distinct mechanisms involving nuclear factor κB. J. Biol. Chem. 268, 5032–5039 (1993).
Heyninck, K. et al. The zinc finger protein A20 inhibits TNF-induced NF-κB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-κB-inhibiting protein ABIN. J. Cell Biol. 145, 1471–1482 (1999).
Heyninck, K. & Beyaert, R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-κB activation at the level of TRAF6. FEBS Lett. 442, 147–150 (1999).
Lee, E. G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000). The first in vivo evidence that A20 is essential for terminating NF-κB signalling.
Opipari, A. W. Jr, Boguski, M. S. & Dixit, V. M. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J. Biol. Chem. 265, 14705–14708 (1990).
Song, H. Y., Rothe, M. & Goeddel, D. V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl Acad. Sci. USA 93, 6721–6725 (1996).
Makarova, K. S., Aravind, L. & Koonin, E. V. A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem. Sci. 25, 50–52 (2000). The initial description of the OTU domain as a highly conserved cysteine protease.
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004). This work identified RIP as an A20 substrate, characterized the ubiquitin ligase activity of A20 and introduced the concept of ubiquitin editing.
Chen, Z. J., Parent, L. & Maniatis, T. Site-specific phosphorylation of IkBa by a novel ubiquitination-dependent protein kinase activity. Cell 84, 853–862 (1996). The first report indicating that a unique form of polyubiquitylation that does not promote substrate degradation activates kinases in NF-κB signalling cascades.
Jackson, P. K. et al. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10, 429–439 (2000).
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001).
Weissman, A. M. Themes and variations on ubiquitylation. Nature Rev. Mol. Cell Biol. 2, 169–178 (2001).
Chiu, Y. H., Zhao, M. & Chen, Z. J. Ubiquitin in NF-κB signaling. Chem. Rev. 109, 1549–1560 (2009).
Reyes-Turcu, F. E., Ventii, K. H. & Wilkinson, K. D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 (2009).
Balakirev, M. Y., Tcherniuk, S. O., Jaquinod, M. & Chroboczek, J. Otubains: a new family of cysteine proteases in the ubiquitin pathway. EMBO Rep. 4, 517–522 (2003).
Borodovsky, A. et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem. Biol. 9, 1149–1159 (2002).
Evans, P. C. et al. A novel type of deubiquitinating enzyme. J. Biol. Chem. 278, 23180–23186 (2003).
Evans, P. C. et al. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem. J. 378, 727–734 (2004).
Lin, S. C. et al. Molecular basis for the unique deubiquitinating activity of the NF-κB inhibitor A20. J. Mol. Biol. 376, 526–540 (2008).
Komander, D. & Barford, D. Structure of the A20 OTU domain and mechanistic insights into deubiquitination. Biochem. J. 409, 77–85 (2008).
Boone, D. L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nature Immunol. 5, 1052–1060 (2004).
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998).
Naito, A. et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4, 353–362 (1999).
Lomaga, M. A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Legler, D. F., Micheau, O., Doucey, M. A., Tschopp, J. & Bron, C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα-mediated NF-κB activation. Immunity 18, 655–664 (2003).
Deng, L. et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 (2000).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Xia, Z. P. et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 (2009).
Xu, M,. Skaug, B,. Zeng, W. & Chen, Z. J. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFα and IL-1β. Mol. Cell 36, 302–314 (2009).
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Newton, K. et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678 (2008). Characterization of endogenous RIP and IRAK polyubiquitylation in activated NF-κB signalling complexes using ubiquitin linkage-specific antibodies.
Hutti, J. E. et al. IkappaB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB pathway. Mol. Cell. Biol. 27, 7451–7461 (2007).
Klinkenberg, M,. Van Huffel, S,. Heyninck, K. & Beyaert, R. Functional redundancy of the zinc fingers of A20 for inhibition of NF-kB activation and protein-protein interactions. FEBS Lett. 498, 93–97 (2001). A detailed characterization of the NF-κB-inhibitory functions of the A20 zinc fingers.
Hsu, H., Huang, J., Shu, H. B., Baichwal, V. & Goeddel, D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Zhang, S. Q., Kovalenko, A., Cantarella, G. & Wallach, D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12, 301–311 (2000). This work showed that RIP is post-translationally modified on TNFR1 recruitment and that modified RIP recruits both NEMO and A20 to the activated signalling complex.
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Mattera, R., Tsai, Y. C., Weissman, A. M. & Bonifacino, J. S. The Rab5 guanine nucleotide exchange factor Rabex-5 binds ubiquitin (Ub) and functions as a Ub ligase through an atypical Ub-interacting motif and a zinc finger domain. J. Biol. Chem. 281, 6874–6883 (2006).
Lee, S. et al. Structural basis for ubiquitin recognition and autoubiquitination by Rabex-5. Nature Struct. Mol. Biol. 13, 264–271 (2006). References 60 and 61 show that the A20-like ZnF in RABGEF1 has intrinsic ubiquitin ligase activity.
Penengo, L. et al. Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin. Cell 124, 1183–1195 (2006).
Sato, Y. et al. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 28, 2461–2468 (2009).
Kulathu, Y., Akutsu, M., Bremm, A., Hofmann, K. & Komander, D. Two-sided ubiquitin binding explains specificity of the TAB2 NZF domain. Nature Struct. Mol. Biol. 16, 1328–1330 (2009).
Shembade, N. et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nature Immunol. 9, 254–262 (2008).
Shembade, N., Parvatiyar, K., Harhaj, N. S. & Harhaj, E. W. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-κB signalling. EMBO J. 28, 513–522 (2009).
Shi, C. S. & Kehrl, J. H. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 278, 15429–15434 (2003).
Shembade, N., Ma, A. & Harhaj, E. W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Lee, T. H., Shank, J., Cusson, N. & Kelliher, M. A. The kinase activity of Rip1 is not required for TNF-α-induced IκB or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 279, 33185–33191 (2004).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Turer, E. E. et al. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J. Exp. Med. 205, 451–464 (2008).
Hitotsumatsu, O. et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity 28, 381–390 (2008).
Jin, Z. et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 137, 721–735 (2009).
Lin, R. et al. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 281, 2095–2103 (2006).
Coornaert, B., Carpentier, I. & Beyaert, R. A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 284, 8217–8221 (2009).
Li, L., Soetandyo, N., Wang, Q. & Ye, Y. The zinc finger protein A20 targets TRAF2 to the lysosomes for degradation. Biochim. Biophys. Acta 1793, 346–353 (2009).
Vereecke, L., Beyaert, R. & van Loo, G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 30, 383–391 (2009).
Honma, K. et al. TNFAIP3 is the target gene of chromosome band 6q23.3-q24.1 loss in ocular adnexal marginal zone B cell lymphoma. Genes Chromosom. Cancer 47, 1–7 (2008).
Novak, U. et al. The NF-κB negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 113, 4918–4921 (2009).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-κB in diffuse large B-cell lymphoma. Nature 459, 717–721 (2009).
Kato, M. et al. Frequent inactivation of A20 in B-cell lymphomas. Nature 459, 712–716 (2009).
Honma, K. et al. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 114, 2467–2475 (2009).
Chanudet, E. et al. A20 deletion is associated with copy number gain at the TNFA/B/C locus and occurs preferentially in translocation-negative MALT lymphoma of the ocular adnexa and salivary glands. J. Pathol. 217, 420–430 (2009).
Chanudet, E. et al. A20 is targeted by promoter methylation, deletion and inactivating mutation in MALT lymphoma. Leukemia 24, 483–487.
Schmitz, R. et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J. Exp. Med. 206, 981–989 (2009).
Schumacher, M. A. et al. Mutations in the genes coding for the NF-κB regulating factors IkBa and A20 are uncommon in nodular lymphocyte-predominant Hodgkin's lymphoma. Haematologica 95, 153–157.
Thome, M. Multifunctional roles for MALT1 in T-cell activation. Nature Rev. Immunol. 8, 495–500 (2008).
Li, L. et al. Localization of A20 to a lysosome-associated compartment and its role in NFκB signaling. Biochim. Biophys. Acta 1783, 1140–1149 (2008).
Vucic, D. & Fairbrother, W. J. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin. Cancer Res. 13, 5995–6000 (2007).
Packham, G. The role of NF-κB in lymphoid malignancies. Br. J. Haematol. 143, 3–15 (2008).
Deshaies, R. J. & Joazeiro, C. A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434 (2009).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Baker, K. P. et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 48, 3253–3265 (2003).
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009).
Schulman, B. A. & Harper, J. W. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nature Rev. Mol. Cell Biol. 10, 319–331 (2009).
Iwai, K. & Tokunaga, F. Linear polyubiquitination: a new regulator of NF-κB activation. EMBO Rep. 10, 706–713 (2009).
Xu, P. et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145 (2009).
Kirkpatrick, D. S. et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biol. 8, 700–710 (2006).
Jin, L., Williamson, A., Banerjee, S., Philipp, I. & Rape, M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell 133, 653–665 (2008).
Ikeda, F. & Dikic, I. Atypical ubiquitin chains: new molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series. EMBO Rep. 9, 536–542 (2008).
Reyes-Turcu, F. E. & Wilkinson, K. D. Polyubiquitin binding and disassembly by deubiquitinating enzymes. Chem. Rev. 109, 1495–1508 (2009).
Mauro, C. et al. ABIN-1 binds to NEMO/IKKγ and co-operates with A20 in inhibiting NF-κB. J. Biol. Chem. 281, 18482–18488 (2006).
Duwel, M. et al. A20 negatively regulates T cell receptor signaling to NF-κB by cleaving Malt1 ubiquitin chains. J. Immunol. 182, 7718–7728 (2009).
Zetoune, F. S. et al. A20 inhibits NF-κB activation downstream of multiple Map3 kinases and interacts with the I kappa B signalosome. Cytokine 15, 282–298 (2001).
Fries, K. L., Miller, W. E. & Raab-Traub, N. The A20 protein interacts with the Epstein-Barr virus latent membrane protein 1 (LMP1) and alters the LMP1/TRAF1/TRADD complex. Virology 264, 159–166 (1999).
Colland, F. et al. Functional proteomics mapping of a human signaling pathway. Genome Res. 14, 1324–1332 (2004).
De Valck, D. et al. The zinc finger protein A20 interacts with a novel anti-apoptotic protein which is cleaved by specific caspases. Oncogene 18, 4182–4190 (1999).
Wang, Y. Y., Li, L., Han, K. J., Zhai, Z. & Shu, H. B. A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-κB and ISRE and IFN-β promoter. FEBS Lett. 576, 86–90 (2004).
Acknowledgements
The authors would like to thank I. Bosanac for insightful discussions and V. Dixit for continued enthusiasm about A20-related proteins.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- A20 Cys2–Cys2 zinc finger (ZnF) motif
-
This motif is found in all eukaryotes, especially in proteins involved in ubiquitin signalling pathways. It is a conserved ubiquitin binding module that functions in diverse biological contexts.
- Ovarian tumour (OTU) domain
-
The OTU domain was first identified in an ovarian tumour gene from Drosophila melanogaster, and is now recognized as a highly conserved domain. Functional and structural studies have confirmed that OTU domains function as deubiquitylases using a conserved cysteine protease fold.
Rights and permissions
About this article
Cite this article
Hymowitz, S., Wertz, I. A20: from ubiquitin editing to tumour suppression. Nat Rev Cancer 10, 332–341 (2010). https://doi.org/10.1038/nrc2775
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2775
This article is cited by
-
A20 promotes colorectal cancer immune evasion by upregulating STC1 expression to block “eat-me” signal
Signal Transduction and Targeted Therapy (2023)
-
A20 interacts with mTORC2 to inhibit the mTORC2/Akt/Rac1 signaling axis in hepatocellular carcinoma cells
Cancer Gene Therapy (2022)
-
Trauma-induced regulation of VHP-1 modulates the cellular response to mechanical stress
Nature Communications (2021)
-
OTUD5 promotes innate antiviral and antitumor immunity through deubiquitinating and stabilizing STING
Cellular & Molecular Immunology (2021)
-
Association of rs610604 in TNFAIP3 and rs17728338 in TNIP1 gene polymorphisms with psoriasis susceptibility: a meta-analysis of case-control studies
BMC Medical Genetics (2020)