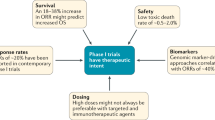
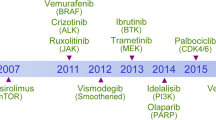
Abstract
The development of novel molecularly targeted cancer therapeutics remains slow and expensive with many late-stage failures. There is an urgent need to accelerate this process by improving early clinical anticancer drug evaluation through modern and rational trial designs that incorporate predictive, pharmacokinetic, pharmacodynamic, pharmacogenomic and intermediate end-point biomarkers. In this article, we discuss current approaches and propose strategies that will potentially maximize benefit to patients and expedite the regulatory approvals of new anticancer drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stratton, M. R., Campbell, P. J. & Futreal, P. A. The cancer genome. Nature 458, 719–724 (2009).
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nature Med. 10, 789–799 (2004).
Collins, I. & Workman, P. New approaches to molecular cancer therapeutics. Nature Chem. Biol. 2, 689–700 (2006).
Iorns, E., Lord, C. J., Turner, N. & Ashworth, A. Utilizing RNA interference to enhance cancer drug discovery. Nature Rev. Drug Discov. 6, 556–568 (2007).
Taube, S. E. et al. A perspective on challenges and issues in biomarker development and drug and biomarker codevelopment. J. Natl. Cancer Inst. 101, 1453–1463 (2009).
McDermott, U. & Settleman, J. Personalized cancer therapy with selective kinase inhibitors: an emerging paradigm in medical oncology. J. Clin. Oncol. 27, 5650–5659 (2009).
Janne, P. A., Gray, N. & Settleman, J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nature Rev. Drug Discov. 8, 709–723 (2009).
Workman, P. & de Bono, J. Targeted therapeutics for cancer treatment: major progress towards personalised molecular medicine. Curr. Opin. Pharmacol. 8, 359–362 (2008).
DiMasi, J. A. & Grabowski, H. G. Economics of new oncology drug development. J. Clin. Oncol. 25, 209–216 (2007).
Reichert, J. M. & Wenger, J. B. Development trends for new cancer therapeutics and vaccines. Drug Discov. Today 13, 30–37 (2008).
DiMasi, J. A., Feldman, L., Seckler, A. & Wilson, A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin. Pharmacol. Ther. 87, 272–277.
Kelloff, G. J. & Sigman, C. C. New science-based endpoints to accelerate oncology drug development. Eur. J. Cancer 41, 491–501 (2005).
Simon, R. The use of genomics in clinical trial design. Clin. Cancer Res. 14, 5984–5993 (2008).
Sarker, D. & Workman, P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv. Cancer Res. 96, 213–268 (2007).
Adjei, A. A. What is the right dose? The elusive optimal biologic dose in Phase I clinical trials. J. Clin. Oncol. 24, 4054–4055 (2006).
Goulart, B., Roberts, T. & Clark, J. Utility and costs of surrogate endpoints (SEs) and biomarkers in Phase I oncology trials. J. Clin. Oncol. 22, (Suppl. 14), 6012 (abstract) (2004).
Yap, T. A. et al. Phase I trial of the irreversible ErbB1 (EGFR) and ErbB2 (HER2) kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J. Clin. Oncol. (in the press).
Huang, R. S. & Ratain, M. J. Pharmacogenetics and pharmacogenomics of anticancer agents. CA Cancer J. Clin. 59, 42–55 (2009).
Walko, C. M. & McLeod, H. Pharmacogenomic progress in individualized dosing of key drugs for cancer patients. Nature Clin. Pract. Oncol. 6, 153–162 (2009).
Workman, P. Challenges of PK/PD measurements in modern drug development. Eur. J. Cancer 38, 2189–2193 (2002).
Workman, P. How much gets there and what does it do? The need for better pharmacokinetic and pharmacodynamic endpoints in contemporary drug discovery and development. Curr. Pharm. Des 9, 891–902 (2003).
Workman, P. Auditing the pharmacological accounts for Hsp90 molecular chaperone inhibitors: unfolding the relationship between pharmacokinetics and pharmacodynamics. Mol. Cancer Ther. 2, 131–138 (2003).
Banerji, U. et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J. Clin. Oncol. 23, 4152–4161 (2005).
Banerji, U. et al. Pharmacokinetic–pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin. Cancer Res. 11, 7023–7032 (2005).
Attard, G., Reid, A. H., Olmos, D. & de Bono, J. S. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 69, 4937–4940 (2009).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Yap, T. A., Carden, C. P., Attard, G. & de Bono, J. S. Targeting CYP17: established and novel approaches in prostate cancer. Curr. Opin. Pharmacol. 8, 449–457 (2008).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Fong, P. C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 20 Apr 2010 (doi: JCO.2009.26.9589v1).
Mandrekar, S. J. & Sargent, D. J. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J. Clin. Oncol. 27, 4027–4034 (2009).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Baselga, J. et al. Objective response rate in a Phase II multicenter trial of pertuzumab (P), a HER2 dimerization inhibiting monoclonal antibody, in combination with trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) which has progressed during treatment with, T.. J. Clin. Oncol. 25, (Suppl. 18), 1004 (abstract) (2007).
Vogel, C. L. et al. A Phase II study of trastuzumab-DM1 (T-DM1), a HER2 antibody–drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC): final results. J. Clin. Oncol. 27 (Suppl. 15), 1017 (abstract) (2009).
Spector, N. L. et al. EGF103009, a Phase II trial of lapatinib monotherapy in patients with relapsed/refractory inflammatory breast cancer (IBC): clinical activity and biologic predictors of response. J. Clin. Oncol. 24 (Suppl. 18S), 502 (abstract) (2006).
Talpaz, M. et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a Phase 2 study. Blood 99, 1928–1937 (2002).
Kwak, E. et al. Clinical activity observed in a Phase I dose escalation trial of an oral c-met and ALK inhibitor, PF-02341066. J. Clin. Oncol. 27 (Suppl. 15), 3509 (abstract) (2009).
Flaherty, K. et al. Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J. Clin. Oncol. 27 (Suppl. 15), 9000 (abstract) (2009).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–34 (2008).
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa survival evaluation in lung cancer). Lancet 366, 1527–1537 (2005).
Takano, T. et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J. Clin. Oncol. 26, 5589–5595 (2008).
Van Cutsem, E. et al. Randomized Phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): the CRYSTAL trial. J. Clin. Oncol. 25 (Suppl. 18), 4000 (abstract) (2007).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Wilhelm, S. M. et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 7, 3129–3140 (2008).
Wilhelm, S. M. et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Hoering, A., Leblanc, M. & Crowley, J. J. Randomized Phase III clinical trial designs for targeted agents. Clin. Cancer Res. 14, 4358–4367 (2008).
Yap, T. A. et al. Targeting the PI3K–AKT–mTOR pathway: progress, pitfalls, and promises. Curr. Opin. Pharmacol. 8, 393–412 (2008).
Workman, P. Clarke, P. A., Raynaud, F. I. & van Montfort, R. L. Drugging the PI3 kinome : from chemical tools to drugs in the clinic. Cancer Res. 70, 2146–2157 (2010).
Comoglio, P. M., Giordano, S. & Trusolino, L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nature Rev. Drug Discov. 7, 504–516 (2008).
Yap, T. A. & de Bono, J. S. Targeting the HGF/c-Met axis: state of play. Mol. Cancer Ther. 9, 1077–1079 (2010).
Burzykowski, T. & Buyse, M. Surrogate threshold effect: an alternative measure for meta-analytic surrogate endpoint validation. Pharm. Stat. 5, 173–186 (2006).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Hou, J. M. et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am. J. Pathol. 175, 808–816 (2009).
Hodgson, D. R. et al. Circulating tumour-derived predictive biomarkers in oncology. Drug Discov. Today 15, 98–101 (2010).
Yerushalmi, R., Woods, R., Ravdin, P. M., Hayes, M. M. & Gelmon, K. A. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 11, 174–183 (2010).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Attard, G. et al. Characterization of, ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918 (2009).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Frese, K. K. & Tuveson, D. A. Maximizing mouse cancer models. Nature Rev. Cancer 7, 645–658 (2007).
Raynaud, F. I. et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol. Cancer Ther. 8, 1725–1738 (2009).
Guillard, S. et al. Molecular pharmacology of phosphatidylinositol 3-kinase inhibition in human glioma. Cell Cycle 8, 443–453 (2009).
Sarker, D. et al. A phase I study evaluating the pharmacokinetics (PK) and pharmacodynamics (PD) of the oral pan-phosphoinositide-3 kinase (PI3K) inhibitor GDC-0941. J. Clin. Oncol. 27 (Suppl. 15), 3358 (abstract) (2009).
Wagner, A. et al. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J. Clin. Oncol. 27 (Suppl. 15), 3501 (abstract) (2009).
Banerji, U., de Bono, J., Judson, I., Kaye, S. & Workman, P. Biomarkers in early clinical trials: the committed and the skeptics. Clin. Cancer Res. 14, 2512 (2008).
Ratain, M. J. & Glassman, R. H. Biomarkers in Phase I oncology trials: signal, noise, or expensive distraction? Clin. Cancer Res. 13, 6545–6548 (2007).
Goulart, B. H. et al. Trends in the use and role of biomarkers in Phase I oncology trials. Clin. Cancer Res. 13, 6719–6726 (2007).
Sawyers, C. L. The cancer biomarker problem. Nature 452, 548–552 (2008).
Park, J. W. et al. Rationale for biomarkers and surrogate end points in mechanism-driven oncology drug development. Clin. Cancer Res. 10, 3885–3896 (2004).
Eisenhauer, E. A., O'Dwyer, P. J., Christian, M. & Humphrey, J. S. Phase I clinical trial design in cancer drug development. J. Clin. Oncol. 18, 684–692 (2000).
Verweij, J., Eskens, F. & de Jonge, M. The multi-institutional Phase I study: disadvantages without advantages? J. Clin. Oncol. 26, 1915–1916 (2008).
Dowlati, A. et al. Multi-institutional Phase I trials of anticancer agents. J. Clin. Oncol. 26, 1926–1931 (2008).
Thomas, R. K. et al. High-throughput oncogene mutation profiling in human cancer. Nature Genet. 39, 347–351 (2007).
Yap, T. A., Carden, C. P. & Kaye, S. B. Beyond chemotherapy: targeted therapies in ovarian cancer. Nature Rev. Cancer 9, 167–181 (2009).
Dancey, J. E., Espinoza-Delgado, I., Papaconstantinou, A., Saunders, J. & Rubinstein, L. Safety, efficacy and efficiency of Phase 1 single agent trials using the accelerated titration (ATD) versus modified Fibonacci designs (STD) in 20th Annual AACR–NCI–EORTC International Conference: Molecular Targets and Cancer Therapeutics, A98 (abstract) (American Association for Cancer Research, Boston, 2009).
Iasonos, A., Wilton, A. S., Riedel, E. R., Seshan, V. E. & Spriggs, D. R. A comprehensive comparison of the continual reassessment method to the standard 3 + 3 dose escalation scheme in Phase I dose-finding studies. Clin. Trials 5, 465–477 (2008).
O'Quigley, J., Pepe, M. & Fisher, L. Continual reassessment method: a practical design for Phase 1 clinical trials in cancer. Biometrics 46, 33–48 (1990).
Sleijfer, S. & Wiemer, E. Dose selection in Phase I studies: why we should always go for the top. J. Clin. Oncol. 26, 1576–1578 (2008).
Booth, C. M. et al. Endpoints and other considerations in Phase I studies of targeted anticancer therapy: recommendations from the task force on Methodology for the Development of Innovative Cancer Therapies (MDICT). Eur. J. Cancer 44, 19–24 (2008).
Propper, D. J. et al. Use of positron emission tomography in pharmacokinetic studies to investigate therapeutic advantage in a Phase I study of 120-hour intravenous infusion XR5000. J. Clin. Oncol. 21, 203–210 (2003).
Turk, D. & Szakacs, G. Relevance of multidrug resistance in the age of targeted therapy. Curr. Opin. Drug Discov. Devel 12, 246–252 (2009).
Workman, P. et al. Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing clinical trials of innovative therapies. J. Natl. Cancer Inst. 98, 580–598 (2006).
Haluska, P. et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751871 in patients with refractory solid tumors. Clin. Cancer Res. 13, 5834–5840 (2007).
Takimoto, C. H. Maximum tolerated dose: clinical endpoint for a bygone era? Target Oncol. 4, 143–147 (2009).
Tutt, A. et al. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer. J. Clin. Oncol. 27 (Suppl. 18), CRA501 (abstract) (2009).
Ratain, M. J. & Sargent, D. J. Optimising the design of Phase II oncology trials: the importance of randomisation. Eur. J. Cancer 45, 275–280 (2009).
Seymour, L. et al. The design of Phase II clinical trials testing cancer therapeutics: consensus recommendations from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin. Cancer Res. 16, 1764–1769.
Tang, H. et al. Comparison of error rates in single-arm versus randomized Phase II cancer clinical trials. J. Clin. Oncol. 28, 1936–1941.
Rubinstein, L., Crowley, J., Ivy, P., Leblanc, M. & Sargent, D. Randomized Phase II designs. Clin. Cancer Res. 15, 1883–1890 (2009).
Workman, P. & Travers, J. Cancer: drug-tolerant insurgents. Nature 464, 844–845 (2010).
Taylor, I. W. et al. Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nature Biotechnol. 27, 199–204 (2009).
Schadt, E. E., Friend, S. H. & Shaywitz, D. A. A network view of disease and compound screening. Nature Rev. Drug Discov. 8, 286–295 (2009).
Gutman, S. & Kessler, L. G. The US Food and Drug Administration perspective on cancer biomarker development. Nature Rev. Cancer 6, 565–571 (2006).
Sarker, D., Pacey, S. & Workman, P. Use of pharmacokinetic/pharmacodynamic biomarkers to support rational cancer drug development. Biomarkers Med. 1, 399–417 (2007).
Clarke, P. A. et al. Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene 19, 4125–4133 (2000).
Hostein, I., Robertson, D., DiStefano, F., Workman, P. & Clarke, P. A. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 61, 4003–4009 (2001).
Tan, D. S. et al. Biomarker-driven early clinical trials in oncology: a paradigm shift in drug development. Cancer J. 15, 406–420 (2009).
Ashworth, A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 26, 3785–3790 (2008).
Audeh, M. et al. Phase II trial of the oral PARP inhibitor olaparib (AZD2281) in BRCA-deficient advanced ovarian cancer. J. Clin. Oncol. 27 (Suppl. 15), 5500 (abstract) (2009).
Kurzrock, R. et al. Project Zero Delay: a process for accelerating the activation of cancer clinical trials. J. Clin. Oncol. 27, 4433–4440 (2009).
Parulekar, W. R. & Eisenhauer, E. A. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J. Natl. Cancer Inst. 96, 990–997 (2004).
Kummar, S., Gutierrez, M., Doroshow, J. H. & Murgo, A. J. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br. J. Clin. Pharmacol. 62, 15–26 (2006).
Le Tourneau, C., Lee, J. J. & Siu, L. L. Dose escalation methods in Phase I cancer clinical trials. J. Natl. Cancer Inst. 101, 708–720 (2009).
Cannistra, S. A. Challenges and pitfalls of combining targeted agents in Phase I studies. J. Clin. Oncol. 26, 3665–3667 (2008).
Krop, I. E. et al. Phase I study of trastuzumab–DM1, an HER2 antibody–drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J. Clin. Oncol. 28, 2698–2704 (2010).
Acknowledgements
The Drug Development Unit of the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research is supported in part by a programme grant from Cancer Research UK. Support was also provided by the Experimental Cancer Medicine Centre (to The Institute of Cancer Research) and the National Institute for Health Research Biomedical Research Centre (jointly to the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research). T.A.Y. is a Cancer Research UK Clinical Research Fellow and P.W. is a Cancer Research UK Life Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All the authors are employees of the Institute of Cancer Research, which has a commercial interest in the development of inhibitors of HSP90, PI3K, AKT, BRAF, PARP, CYP17, CDK and chromatin-modifying enzymes. The authors have potentially relevant commercial interactions with Vernalis Ltd, Novartis, Piramed Pharma (acquired by Roche), Astex Therapeutics, AstraZeneca, GSK, Cougar Biotechnology Inc. (acquired by Johnson & Johnson), Merck Serona and Cyclacel Pharmaceuticals.
Related links
Related links
DATABASES
clinicaltrials.gov
National Cancer Institute Drug Dictionary
Glossary
- Biologically active dose range
-
The range of drug doses required to result in the modulation of the cellular target of the drug to produce its expected effect.
- Continual reassessment method
-
This tool uses statistical modelling and is employed in dose-finding clinical trials to estimate the dose at which the desired toxicity level can be expected to minimize risk of toxicity to patients.
- Maximum tolerated dose
-
The highest dose of a drug or treatment that does not cause unacceptable side effects.
- Pharmacodynamics
-
The relationship between drug concentration and its biological effects (what the drug does to the body).
- Pharmacogenetics
-
This term was coined in 1959 and represents the study of genetic factors that influence response to drugs and chemicals18.
- Pharmacogenomics
-
Recent advances and improvements in large genome-scale sequencing and bioinformatic tools for processing data have led to the transition of pharmacogenetics to pharmacogenomics, which involves studies of the entire spectrum of genes in the human genome18.
- Pharmacokinetics
-
The concentration of drugs in the body over a period of time, including the processes by which drugs are absorbed, distributed in the body, localized in tissues, metabolized and excreted (what the body does to the drug).
- Predictive biomarker
-
Any measurement associated with response to or lack of response to a particular therapy.
- Response Evaluation Criteria In Solid Tumours
-
A set of published rules that define when cancer patients improve (respond), stay the same (stable) or worsen (progress) during treatments.
- Single-arm Phase II trial
-
A trial that demonstrates the safety and activity of a drug in a selected group of patients. This is in contrast to randomized clinical trials, which involve the random allocation of different treatments (including placebo) to patients in different groups.
- Surrogate threshold effect
-
The minimum treatment effect on the surrogate end point necessary to predict a non-zero effect on the true end point.
- Synthetic lethality
-
In genetics, a phenomenon in which the combination of two otherwise non-lethal mutations results in a non-viable cell.
Rights and permissions
About this article
Cite this article
Yap, T., Sandhu, S., Workman, P. et al. Envisioning the future of early anticancer drug development. Nat Rev Cancer 10, 514–523 (2010). https://doi.org/10.1038/nrc2870
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2870
This article is cited by
-
A review on reported phytochemicals as druggable leads with antimalarial potential
Medicinal Chemistry Research (2023)
-
New strategy for antimetastatic treatment of lung cancer: a hypothesis based on circulating tumour cells
Cancer Cell International (2022)
-
Determining drug dose in the era of targeted therapies: playing it (un)safe?
Blood Cancer Journal (2022)
-
Precision oncology in metastatic colorectal cancer — from biology to medicine
Nature Reviews Clinical Oncology (2021)
-
Randomised Phase 1 clinical trials in oncology
British Journal of Cancer (2021)