Key Points
-
The availability of high-energy beams of photons, protons and carbon ions has contributed to increases in tumour control and the sparing of normal tissues from acute radiation toxicity.
-
Advances in cancer therapies for children have produced impressive prospects for long-term survival. Approximately 80% of children and adolescents treated for cancer survive more than 5 years, but roughly 73% of them develop treatment-related complications. The complication of perhaps greatest concern is the risk for developing a radiogenic second malignant neoplasm (SMN), which can develop years or decades after treatment.
-
Although the patient receives a high dose of therapeutic radiation, which is focused at the diseased tissue, the entire body is exposed to comparatively low doses of unwanted radiation that are caused by radiation leaking from the treatment apparatus and by scattering of the therapeutic radiation within the body.
-
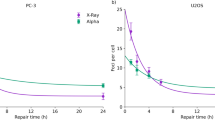
Mechanisms of therapy-related cancers are similar to those of sporadic tumorigenesis, but the carcinogenic potential of low doses of photons is not completely understood, and the uncertainty is much higher for cancer that is induced by charged particles.
-
Epidemiological studies have conclusively shown that some SMNs can develop in tissues that are located in-field (that is, in the path of the therapeutic beam) and out-of-field (outside the path of the therapeutic beam).
-
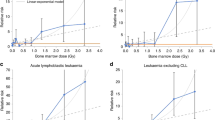
Recent models predict that particle therapy lowers the risk of SMNs compared with contemporary photon therapies.
-
Regardless of the type of radiation beams used, nascent approaches to personalized, risk-adapted radiotherapy seem to be likely to yield further reductions in risk from out-of-field exposures, and research in genetic susceptibility and radiobiology should help to identify biomarkers of long-term risk in cancer survivors.
Abstract
Recent advances in radiotherapy have enabled the use of different types of particles, such as protons and heavy ions, as well as refinements to the treatment of tumours with standard sources (photons). However, the risk of second cancers arising in long-term survivors continues to be a problem. The long-term risks from treatments such as particle therapy have not yet been determined and are unlikely to become apparent for many years. Therefore, there is a need to develop risk assessments based on our current knowledge of radiation-induced carcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Smith, M. A. et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J. Clin. Oncol. 28, 2625–2634 (2010).
Friedman, D. L. et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 102, 1083–1095 (2010). These are the most recent results of the CCSS, the most important epidemiological analysis of SMNs in children.
Oeffinger, K. C. et al. for the Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N. Eng. J. Med. 355, 1572–1582 (2006).
Robison, L. L. et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J. Clin. Oncol. 27, 2308–2318 (2009).
West, C. & Rosenstein, B. S. Establishment of a radiogenomics consortium. Radiother. Oncol. 94, 117–118 (2010).
Travis, L. B. et al. Cancer survivorship--genetic susceptibility and second primary cancers: research strategies and recommendations. J. Natl. Cancer Inst. 98, 15–25 (2006).
Tubiana, M. Can. we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother. Oncol. 91, 4–15 (2009).
Meropol, N. J. & Schulman, K. A. Cost of cancer care: issues and implications. J. Clin. Oncol. 25, 180–186 (2007).
Terasawa, T. et al. Systematic review: charged-particle radiation therapy for cancer. Ann. Intern. Med. 151, 556–565 (2009).
Durante, M. & Loeffler, J. S. Charged particles in radiation oncology. Nature Rev. Clin. Oncol. 7, 37–43 (2010). A review of the open research topics in particle therapy, including both tumour control and the risk of complications.
Schulz-Ertner, D. & Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 25, 953–964 (2007).
Hall, E. J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 65, 1–7 (2006). This paper started the heated debate on the risk of secondary cancers using IMRT and proton therapy.
Brenner, D. J. & Hall, E. J. Secondary neutrons in clinical proton radiotherapy: a charged issue. Radiother. Oncol. 86, 165–170 (2008).
Newhauser, W. D. Complexity of advanced radiation therapy necessitates multidisciplinary inquiry into dose reconstruction and risk assessment. Phys. Med. Biol. 55, e01 (2010).
Reulen, R. C. et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 304, 172–179 (2010).
Yock, T. I. & Tarbell, N. J. Proton beam radiotherapy for treatment in pediatric brain tumors. Nature Clin. Pract. Oncol. 1, 97–103 (2004).
Combs, S. E. et al. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 115, 1348–1355 (2009).
Merchant, T. E. Proton beam therapy in pediatric oncology. Cancer J. 15, 298–305 (2009).
Armstrong, G. T., Stovall, M. & Robison, L. L. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the Childhood Cancer Survivor Study. Radiat. Res. 174, 840–850 (2010).
Lichter, A. S. & Lawrence, T. S. Recent advances in radiation oncology. N. Engl. J. Med. 332, 371–379 (1995).
Bernier, J., Hall, E. J. & Giaccia, A. Radiation oncology: a century of achievements. Nature Rev. Cancer 4, 737–747 (2004).
Bucci, M. K., Bevan, A. & Roach, M. 3rd. Advances in radiation therapy: conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J. Clin. 55, 117–134 (2005).
Goodhead, D. T. et al. Direct comparison between protons and alpha-particles of the same LET: I. Irradiation methods and inactivation of asynchronous V79, HeLa and C3H 10T1/2 cells. Int. J. Radiat. Biol. 61, 611–624 (1992).
Edwards, A. A. RBE of radiations in space and the implications for space travel. Phys. Med. 17, S147–S152 (2001).
Webb, S. The physical basis of IMRT and inverse planning. Br. J. Radiol. 76, 678–689 (2003).
Bortfeld, T. IMRT: a review and preview. Phys. Med. Biol. 51, R363–R379 (2006).
Verellen, D. et al. Innovations in image-guided radiotherapy. Nature Rev. Cancer 7, 949–960 (2007).
Hall, E. J. & Wuu, C. S. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 56, 83–88 (2003).
Halperin, E. C. Particle therapy and treatment of cancer. Lancet Oncol. 7, 676–685 (2006).
Schardt, D., Elsässer, T. & Schulz-Ertner, D. Heavy-ion tumor therapy: physical and radiobiological benefits. Rev. Mod. Phys. 82, 383–425 (2010).
Durante, M. & Cucinotta, F. A. Heavy ion carcinogenesis and human space exploration. Nature Rev. Cancer 8, 465–472 (2008).
Xu, X. G., Bednarz, B. & Paganetti, H. A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction. Phys. Med. Biol. 53, 193–241 (2008). A comprehensive review that includes a careful comparison of the secondary radiation from different radiotherapy facilities.
Krämer, M. & Durante, M. Ion beam transport calculations and treatment plans in particle therapy. Eur. Phys. J. D60, 195–202 (2010).
Howell, R. M. et al. Methodology for determining doses to in-field, out-of-field and partially in-field organs for late effects studies in photon radiotherapy. Phys. Med. Biol. 55, 7009–7023 (2010).
Stovall, M. et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat. Res. 166, 141–157 (2006).
Newhauser, W. D. et al. Can megavoltage computed tomography reduce proton range uncertainties in treatment plans for patients with large metal implants? Phys. Med. Biol. 53, 2327–2344 (2008).
Schneider, U. et al. Secondary neutron dose during proton therapy using spot scanning Int. J. Radiat. Oncol. Biol. Phys. 53, 244–251 (2002).
Gunzert-Marx, K. et al. Secondary beam fragments produced by 200 MeVu−112C ions in water and their dose contributions in carbon ion radiotherapy. New J. Phys. 10, 075003 (2008). A comprehensive set of measurements on neutrons produced by heavy ion therapy.
Yonai, S. et al. Measurement of absorbed dose, quality factor, and dose equivalent in water phantom outside of the irradiation field in passive carbon-ion and proton radiotherapies. Med. Phys. 37, 4046–4055 (2010).
Münter, M. et al. Heavy ion radiotherapy during pregnancy. Fertil. Steril. 94, 2329.e5–7 (2010). Direct evidence from a pregnant patient that particle therapy produced low levels of stray radiation in distal organs.
ICRU. Prescribing, recording, and reporting proton-beam therapy. ICRU Report No. 78. (J. ICRU, 2007).
Wambersie, A. et al. The RBE issues in ion-beam therapy: conclusions of a joint IAEA/ICRU working group regarding quantities and units. Radiat. Prot. Dosim. 122, 463–470 (2006).
Brenner, D. J. Effective dose: a flawed concept that could and should be replaced. Br. J. Radiol. 81, 521–523 (2008).
ICRP. Recommendations of the ICRP. ICRP Publication 103.) Ann. ICRP 37 2007).
El Ghissassi, F. et al. A review of human carcinogens-part D: radiation. Lancet Oncol. 10, 751–752 (2009).
UNSCEAR. Effects of Ionizing Radiation. Volume I. Annex A: Epidemiological Studies of Radiation and Cancer. (United Nations Office, Vienna, 2006).
Pawel, D. et al. Improved estimates of cancer site-specific risks for A-bomb survivors. Radiat. Res. 169, 87–98 (2008).
Mullenders, L. et al. Assessing cancer risks of low-dose radiation. Nature Rev. Cancer 9, 596–604 (2009).
Schneider, U., Lomax, A. & Timmermann, B. Second cancers in children treated with modern radiotherapy techniques. Radiother. Oncol. 89, 135–140 (2008).
Sachs, R. K. & Brenner, D. J. Solid tumor risks after high doses of ionizing radiation. Proc. Natl Acad. Sci. USA 102, 13040–13045 (2005). An excellent model of risk at high doses, which is important for radiotherapy.
Allan, J. M. & Travis, L. B. Mechanisms of therapy-related carcinogenesis. Nature Rev. Cancer 5, 943–955 (2005).
Little, M. P. Cancer after exposure to radiation in the course of treatment for benign and malignant disease. Lancet Oncol. 2, 212–220 (2001).
Malone, K. K. et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J. Clin. Oncol. 28, 2404–2410 (2010).
Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability-an evolving hallmark of cancer. Nature Rev. Mol. Cell. Biol. 11, 220–228 (2010).
Rosemann, M. et al. Multilocus inheritance determines predisposition to α-radiation induced bone tumourigenesis in mice. Int. J. Cancer 118, 2132–2138 (2006).
Gonzalez-Vasconcellos, I. et al. Differential effects of genes of the Rb1 signalling pathway on osteosarcoma incidence and latency in α-particle irradiated mice. Radiat. Environ. Biophys. 50, 135–141 (2011).
Brenner, D. J. et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA 100, 13761–13766 (2003).
Mothersill, C. & Seymour, C. B. Radiation-induced bystander effects-implications for cancer. Nature Rev. Cancer 4, 158–164 (2004).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Barcellos-Hoff, M. H., Park, C. & Wright, E. G. Radiation and the microenvironment - tumorigenesis and therapy. Nature Rev. Cancer 5, 867–875 (2005).
Ikushima, H. & Myazono, K. TGFβ signalling: a complex web in cancer progression. Nature Rev. Cancer 10, 415–424 (2010).
Shuryak, I., Sachs, R. K. & Brenner, D. J. Cancer risks after radiation exposure in middle age. J. Natl. Cancer Inst. 102, 1628–1636 (2010). An important model of age-dependent cancer risk that suggests that promotion not initiation could be the most important factor in radiation carcinogenesis in adults.
Goss, P. E & Chambers, A. F. Does tumour dormancy offer a therapeutic target? Nature Rev. Cancer 10, 871–877 (2010).
Prise, K. M. & O'Sullivan, J. M. Radiation-induced bystander signalling in cancer therapy. Nature Rev. Cancer 9, 351–360 (2009).
Mancuso, M. et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl Acad. Sci. USA 105, 12445–12450 (2008). Evidence from an animal model that cancer in distal organs can be caused by a non-targeted (bystander) effect and not low dose, stray radiation.
Burr, K. L. et al. Radiation-induced delayed bystander-type effects mediated by hemopoietic cells. Radiat. Res. 173, 760–768 (2010).
Calabrese, E. J. & Baldwin, L. A. Toxicology rethinks its central belief. Nature 421, 691–692 (2003).
Dobbs, T. A. et al. Interplay of two major repair pathways in the processing of complex double-strand DNA breaks. DNA Repair 7, 1372–1383 (2008).
Jakob, B. et al. Live cell microscopy analysis of radiation-induced DNA double-strand break motion. Proc. Natl Acad. Sci. USA 106, 3172–3177 (2009).
Ritter, S. & Durante, M. Heavy-ion induced chromosomal aberrations: a review. Mutat. Res. 701, 38–46 (2010).
Cucinotta, F. A. & Durante, M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol. 7, 431–435 (2006).
Ding, L. H. et al. Gene expression changes in normal human skin fibroblasts induced by HZE-particle radiation. Radiat. Res. 164, 523–526 (2005).
Ding, L. H. et al. Gene expression profiles of normal human fibroblasts after exposure to ionizing radiation: a comparative study of low and high doses. Radiat. Res. 164, 17–26 (2005).
Wood, D. H. Long-term mortality and cancer risk in irradiated rhesus monkeys. Radiat. Res. 126, 132–140 (1991).
Paganetti, H. et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 53, 407–421 (2002).
Alpen, E. L. et al. Tumorigenic potential of high-Z., high-LET charged particle radiations. Radiat. Res. 88, 132–143 (1993).
Dicello, J. F. et al. In vivo mammary tumourigenesis in the Sprague-Dawley rat and microdosimetric correlates. Phys. Med. Biol. 49, 3817–3830 (2004).
Wolf, C. et al. Neutron RBE for induction of tumors with high lethality in Sprague-Dawley rats. Radiat. Res. 154, 412–420 (2000).
Grahn, D., Lombard, L. S. & Carnes, B. A. The comparative tumorigenic effects of fission neutrons and cobalt-60 gamma rays in the B6CF1 mouse. Radiat. Res. 129, 19–36 (1992).
Hollander, C. F., Zurcher, C. & Broerse, J. J. Tumorigenesis in high-dose total body irradiated Rhesus Monkeys – a life span study. Toxicol. Pathol. 31, 209–213 (2003).
Kuhne, W. W. et al. Biological effects of high-energy neutrons measured in vivo using a vertebrate model. Radiat. Res. 172, 473–480 (2009).
Weil, M. M. et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon 56Fe ions. Radiat. Res. 172, 213–219 (2009).
Rola, R. et al. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with 56Fe particles. Radiat. Res. 169, 626–632 (2008).
NCRP. Uncertainties in fatal cancer risk estimates used in radiation protection. Report No. 126. (NCRP, Bethesda, USA, 1997).
Suit, H. et al. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat. Res. 167, 12–42 (2007).
Hill, C. K. et al. Fission-spectrum neutrons at reduced dose rates enhance neoplastic transformation. Nature 298, 67–69 (1982).
Brenner, D. J. & Hall, E. J. Commentary 2 to Cox and Little: radiation-induced oncogenic transformation: the interplay between dose, dose protraction, and radiation quality. Adv. Radiat. Biol. 16, 167–179 (1992).
National Research Council. Health Risks from Low Levels of Ionizing Radiation: BEIR VII Phase 2. (National Academies Press, Washington, DC, 2006).
Miralbell, R. et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int. J. Radiat. Oncol. Biol. Phys. 54, 824–829 (2002). The first model of secondary cancers in paediatric patients treated with protons, suggesting that risk should be lower than for X-rays.
Newhauser, W. D. et al. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys. Med. Biol. 54, 2277–2291 (2009).
Fontenot, J. D., Lee, A. K. & Newhauser, W. D. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated X-ray therapy for early-stage prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 74, 616–622 (2009). A direct comparison of predicted SMN risk for IMRT and protons indicating that a lower risk is expected for particles.
Taddei, P. J. et al. Risk of second malignant neoplasm following proton versus intensity-modulated photon radiotherapies for hepatocellular carcinoma. Phys. Med. Biol. 55, 7055–7065 (2010).
Taddei, P. J. et al. Effective dose from stray radiation for a patient receiving proton therapy for liver cancer. AIP Conf. Proc. 1099, 445–449 (2009).
Taddei, P. J. et al. Predicted risks of second malignant neoplasm incidence and mortality due to secondary neutrons in a girl and boy receiving proton craniospinal irradiation. Phys. Med. Biol. 55, 7067–7080 (2010).
Taddei, P. J. et al. Reducing stray radiation dose for a pediatric patient receiving proton cranospinal irradiation. Nucl. Techn. 168, 108–112 (2009). Evidence that simple counter measures can effectively reduce the secondary dose in paediatric patients.
Taddei, P. J. et al. Reducing stray radiation dose to patients receiving passively scattered proton radiotherapy for prostate cancer. Phys. Med. Biol. 53, 2131–2147 (2008).
Ottolenghi, A., Smyth, V. & Trott, K. R. The risk to healthy tissue from the use of existing and emerging techniques for radiation therapy. Radiat. Prot. Dosim. 143, 533–535 (2011).
Brenner, D. J. Medical imaging in the 21st century — getting the best bang for the rad. N. Eng. J. Med. 362, 943–945 (2010).
Brenner, D. J. & Hricak, H. Radiation exposure from medical imaging: time to regulate? JAMA 304, 208–209 (2010).
Green, S. & Aird, E. Imaging in radiotherapy. Br. J. Radiol. 80, 967–969 (2007).
Deng, J. et al. Kilovoltage imaging dose in the radiotherapy of pediatric patients. Int. J. Radiat. Oncol. Biol. Phys. 6 Apr 2011 [e-pub ahead of print].
Chung, C. S. et al. Comparative analysis of secondary malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int. J. Radiat. Oncol. Biol. Phys. 72, S8 (2008).
Durante, M. et al. X-rays vs. carbon-ion tumor therapy: cytogenetic damage in lymphocytes. Int. J. Radiat. Oncol. Biol. Phys. 47, 793–798 (2000).
Hartel, C. et al. Chromosomal aberrations in peripheral blood lymphocytes of prostate cancer patients treated with IMRT and carbon ions. Radiother. Oncol. 95, 73–78 (2010).
Mole, R. H. Dose–response relationships in Radiation Carcinogenesis: Epidemiology and Biologic Significance (eds Boice, J. D. and Fraumeni, J. F.) 263–271 (Raven Press, New York, 1984).
Acknowledgements
This work was supported in part by the US National Cancer Institute (awards 1R01CA131463-01A1) and by Northern Illinois University, USA, through a subcontract of the US Department of Defense (award W81XWH-08-1-0205). Research on particle therapy at GSI is partially supported by EU FP7 (ALLEGRO project), ESA (IBER-project) and Beilstein Stiftung (NanoBiC program). The authors would like to thank K. B. Carnes for her assistance in preparing this manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Medulloblastoma
-
Malignant primary brain tumour that originates in the cerebellum or posterior fossa.
- Atomic bomb survivors database
-
Survivors of the atomic bombs dropped in 1945 in Japan have been followed for cancers for more than 60 years, and represent the main source of epidemiological data on radiogenic cancers.
- Stray radiation
-
Therapeutic beam radiation that is emitted through the accelerator housing and reaches the patient outside of the treatment volume.
- Radiation quality
-
Ionizing radiation includes many different qualities: high-energy electromagnetic radiation (such as X-rays), neutrons, electrons, protons and heavy ions. Their different biological effectiveness is scaled using weighting factors (Box 1).
- Relative biological effectiveness
-
The ratio of the dose, DR, of a reference radiation (typically γ-ray or X-rays) and Dt of a test radiation (for example, neutrons, protons and heavy ions) that produce the same biological effect. It depends on several factors including the dose, dose rate, biological end point, radiation test LET and tissue.
- α-particles
-
Helium nuclei emitted by some heavy elements by a natural radioactive process known as α-decay. They represent the high-LET component of the natural radiation background for the general population, mostly caused by inhalation of Radon gas.
- Intensity-modulated radiation therapy
-
Currently the most advanced type of photon radiotherapy. Accurate conformation to the target tumour is achieved by increasing the intensity of the rays to the target, and reducing the intensity of the beams that cross sensitive structures. The resulting inhomogenous dose distribution in the single field is compensated by cold and hot spots in the beams coming from other directions.
- Monitor units
-
A monitor unit (MU) is a measure of the linear accelerator (Linac) output. The dose to the target is calibrated with a detector (monitor), and therefore MUs correspond to a given dose to the tumour.
- Bragg peak
-
Region where charged particles release most of their energy in matter, before stopping.
- Passive proton beam shaping
-
A technique that spreads the Bragg peak, using attenuators and collimators.
- Magnetically scanned beams
-
Also known as spot scanning, a technique to deliver particle therapy. A small pencil beam is deflected by a magnet in two dimensions to cover a slice of the tumour, and the next slice is exposed by changing the energy in the accelerator. Unlike passive beam shaping, it does not require attenuators to modulate the Bragg peak, and therefore the production of neutrons outside the patient's body is negligible.
- Linear-no-threshold
-
The model commonly adopted by the International Regulatory Agencies to extrapolate the radiation risk at low doses. It is assumed that the cancer risk is always directly proportional to the absorbed dose.
- Sterilization effects
-
At high radiation doses, the cell killing (sterilization) overcomes the radiation transforming potential, and hence the neoplastic transformation per exposed cell decreases.
- Fractionation
-
The therapeutic radiation dose is very high (up to 60–70 Gy), but is normally delivered in daily fractions of approximately 2 Gy for effective sparing of the normal tissue.
- Ataxia telangectasia
-
A rare and severe neurodegenerative disease (also known as Boder–Sedgwick or Louis–Bar syndrome). It is caused by a defect in ATM, which encodes a serine/threonine protein kinase involved in DNA repair and cell cycle regulation.
- Non-targeted effects
-
Radiation effect observed in cells, tissue or organs not directly exposed to radiation. It can be caused by cell-to-cell communication via gap junctions, release of cytokines in the body, or mediated by the immune or nervous system.
- Bystander effect
-
A non-targeted effect generally observed in cellular experiments. The 'bystander' cell can receive radiation damage, although only the neighbouring target cell is exposed.
- Abscopal effect
-
A radiation response in an organ not directly exposed to the radiation field.
- Harderian gland
-
A subcutaneous accessory lacrimal gland found within the eye's orbit in many vertebrates (not in humans).
- Sparing effect
-
When the same radiation dose is delivered in multiple fractions, at intervals of several hours (typically 1 day), the biological damage is generally reduced.
- Fission-spectrum neutrons
-
Neutrons produced during the nuclear fission process, typically in nuclear reactors for energy production. The energy spectrum peaks at about 1 MeV.
Rights and permissions
About this article
Cite this article
Newhauser, W., Durante, M. Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer 11, 438–448 (2011). https://doi.org/10.1038/nrc3069
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3069
This article is cited by
-
Risk and prognosis of secondary esophagus cancer after radiotherapy for breast cancer
Scientific Reports (2023)
-
High-LET charged particles: radiobiology and application for new approaches in radiotherapy
Strahlentherapie und Onkologie (2023)
-
Mitigating Radiotoxicity in the Central Nervous System: Role of Proton Therapy
Current Treatment Options in Oncology (2023)
-
Monte Carlo study of the neutron ambient dose equivalent at the heavy ion medical machine in Wuwei
Nuclear Science and Techniques (2022)
-
Atomically precise silver clusterzymes protect mice from radiation damages
Journal of Nanobiotechnology (2021)