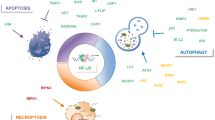
Key Points
-
The nuclear factor-κB (NF-κB)–inhibitor of NF-κB kinase (IKK) pathway can promote the growth and survival of many solid and haematological maligancies and therefore has the potential to provide numerous targets for novel anticancer therapies.
-
Most attention has focused on the development of IKKβ inhibitors, but it is now clear that IKKβ has many NF-κB-independent functions and its inhibition could result in undesired effects.
-
Although it is apparent that NF-κB subunits have important roles in tumorigenesis and the response to cancer therapy, their individual contributions have not been clearly defined.
-
The NF-κB response is highly pleiotropic and the consequences of its activation can be context dependent. NF-κB is not always tumour promoting and it can exhibit tumour suppressor-like activities.
-
Crosstalk with tumour-suppressor proteins, such as p53, provides an important mechanism for regulating NF-κB activity and function in cancer. Tumour suppressors can inhibit the tumour-promoting activities of NF-κB subunits while facilitating their ability to suppress cancer progression.
-
Understanding the regulation and function of the NF-κB subunits in cancer provides opportunities for the development of new therapies and allows the better use of existing drugs that affect NF-κB–IKK activity.
Abstract
It is only recently that the full importance of nuclear factor-κB (NF-κB) signalling to cancer development has been understood. Although much attention has focused on the upstream pathways leading to NF-κB activation, it is now becoming clear that the inhibitor of NF-κB kinases (IKKs), which regulate NF-κB activation, have many independent functions in tissue homeostasis and normal immune function that could compromise the clinical utility of IKK inhibitors. Therefore, if the NF-κB pathway is to be properly exploited as a target for both anticancer and anti-inflammatory drugs, it is appropriate to reconsider the complex roles of the individual NF-κB subunits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nature Immunol. 12, 715–723 (2011).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Gilmore, T. D. & Herscovitch, M. Inhibitors of NF-κB signaling: 785 and counting. Oncogene 25, 6887–6899 (2006).
Baud, V. & Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nature Rev. Drug Discov. 8, 33–40 (2009).
Chariot, A. The NF-κB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 19, 404–413 (2009).
Perkins, N. D. Integrating cell-signalling pathways with NF-κB and IKK function. Nature Rev. Mol. Cell Biol. 8, 49–62 (2007).
Wan, F. & Lenardo, M. J. The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives. Cell Res. 20, 24–33 (2010).
O'Shea, J. M. & Perkins, N. D. Regulation of the RelA (p65) transactivation domain. Biochem. Soc. Trans. 36, 603–608 (2008).
Hayden, M. S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008).
Kanarek, N., London, N., Schueler-Furman, O. & Ben-Neriah, Y. Ubiquitination and degradation of the inhibitors of NF-κB. Cold Spring Harb. Perspect. Biol. 2, a000166 (2010).
Rao, P. et al. IκBβ acts to inhibit and activate gene expression during the inflammatory response. Nature 466, 1115–1119 (2010).
Beinke, S., Robinson, M. J., Hugunin, M. & Ley, S. C. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IκB kinase-induced proteolysis of NF-κB1 p105. Mol. Cell. Biol. 24, 9658–9667 (2004).
Waterfield, M., Jin, W., Reiley, W., Zhang, M. & Sun, S. C. IκB kinase is an essential component of the Tpl2 signaling pathway. Mol. Cell. Biol. 24, 6040–6048 (2004).
Wertz, I. E. & Dixit, V. M. Signaling to NF-κB: regulation by ubiquitination. Cold Spring Harb. Perspect. Biol. 2, a003350 (2010).
Pahl, H. L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18, 6853–6866 (1999).
Kim, H. J., Hawke, N. & Baldwin, A. S. NF-κB and IKK as therapeutic targets in cancer. Cell Death Differ. 13, 738–747 (2006).
Kumar, A., Takada, Y., Boriek, A. M. & Aggarwal, B. B. Nuclear factor-κB: its role in health and disease. J. Mol. Med. 82, 434–448 (2004).
Karin, M. Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 (2006).
Iliopoulos, D., Hirsch, H. A. & Struhl, K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706 (2009).
Fan, Y., Dutta, J., Gupta, N., Fan, G. & Gelinas, C. Regulation of programmed cell death by NF-κB and its role in tumorigenesis and therapy. Adv. Exp. Med. Biol. 615, 223–250 (2008).
Perkins, N. D. & Gilmore, T. D. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 13, 759–772 (2006).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Kawauchi, K., Araki, K., Tobiume, K. & Tanaka, N. p53 regulates glucose metabolism through an IKK-NF-κB pathway and inhibits cell transformation. Nature Cell Biol. 10, 611–618 (2008).
Johnson, R. F., Witzel, II & Perkins, N. D. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-κB. Cancer Res. 71, 5588–5597 (2011).
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Karin, M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 1, a000141 (2009).
Bollrath, J. & Greten, F. R. IKK/NF-κB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 10, 1314–1319 (2009).
Grivennikov, S. I. & Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21, 11–19 (2010).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Harhaj, E. W. & Dixit, V. M. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 21, 22–39 (2011).
Ashall, L. et al. Pulsatile stimulation determines timing and specificity of NF-κB-dependent transcription. Science 324, 242–246 (2009).
Paszek, P. et al. Population robustness arising from cellular heterogeneity. Proc. Natl Acad. Sci. USA 107, 11644–11649 (2010).
Courtois, G. & Gilmore, T. D. Mutations in the NF-κB signaling pathway: implications for human disease. Oncogene 25, 6831–6843 (2006).
Demchenko, Y. N. et al. Classical and/or alternative NF-κB pathway activation in multiple myeloma. Blood 115, 3541–3552 (2010).
Staudt, L. M. Oncogenic activation of NF-κB. Cold Spring Harb. Perspect. Biol. 2, a000109 (2010).
Grivennikov, S. I. & Karin, M. Inflammation and oncogenesis: a vicious connection. Curr. Opin. Genet. Dev. 20, 65–71 (2010).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004).
Luedde, T. et al. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11, 119–132 (2007).
Meylan, E. et al. Requirement for NF-κB signalling in a mouse model of lung adenocarcinoma. Nature 462, 104–107 (2009). This reference shows that NF-κB promotes tumorigenesis in a mouse model of lung adenocarcinoma but that this is dependent on the initial loss of p53.
Basseres, D. S., Ebbs, A., Levantini, E. & Baldwin, A. S. Requirement of the NF-κB subunit p65/RelA for K-Ras-induced lung tumorigenesis. Cancer Res. 70, 3537–3546 (2010). In contrast to reference 40, this reference finds that p53 status does not affect RELA-dependent tumorigenesis, despite also using a KrasG12D model of lung adenocarcinoma. A potential explanation for this is that reference 40 uses a mutant IκBα, which can be expected to inhibit multiple NF-κB complexes, but reference 41 uses a conditional Rela−/− model. These papers underline the complexity of interpreting NF-κB subunit functions in cancer.
Hoffmann, A., Leung, T. H. & Baltimore, D. Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J. 22, 5530–5539 (2003).
Iotsova, V. et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nature Medicine 3, 1285–1289 (1997).
Grossmann, M. et al. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 19, 6351–6360 (2000).
Gilmore, T. D. Multiple myeloma: lusting for NF-κB. Cancer Cell 12, 95–97 (2007).
Greenman, C. et al. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 (2007).
Vlantis, K. et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J. Clin. Invest. 121, 2781–2793 (2011).
Gilmore, T. D. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene 18, 6925–6937 (1999).
Campbell, K. J., Rocha, S. & Perkins, N. D. Active repression of antiapoptotic gene expression by RelA (p65) NF-κB. Mol. Cell 13, 853–865 (2004).
Yang, G. et al. The biphasic role of NF-κB in progression and chemoresistance of ovarian cancer. Clin. Cancer Res. 17, 2181–2194 (2011).
Strozyk, E., Poppelmann, B., Schwarz, T. & Kulms, D. Differential effects of NF-κB on apoptosis induced by DNA-damaging agents: the type of DNA damage determines the final outcome. Oncogene 25, 6239–6251 (2006). References 49 and 51 showed that activation of NF-κB after DNA damage can be pro-apoptotic rather than anti-apoptotic. A prediction of these and other similar papers is that NF-κB activity can vary depending on tumour stage. Reference 50 confirms this hypothesis by showing a biphasic response of NF-κB with a transition from tumour suppressor to tumour promoter.
Perkins, N. D. Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene 25, 6717–6730 (2006).
Huang, B., Yang, X. D., Lamb, A. & Chen, L. F. Posttranslational modifications of NF-κB: another layer of regulation for NF-κB signaling pathway. Cell. Signal 22, 1282–1290 (2010).
Barre, B. & Perkins, N. D. Phosphorylation of the p52 NF-κB subunit. Cell cycle 9, 4774–4775 (2010).
Li, H. et al. Regulation of NF-κB activity by competition between RelA acetylation and ubiquitination. Oncogene, 27 Jun 2011 (doi:10.1038/onc.2011.253).
Zotti, T. et al. TRAF7 protein promotes Lys-29-linked polyubiquitination of IκB kinase (IKKγ)/NF-κB essential modulator (NEMO) and p65/RelA protein and represses NF-κB activation. J. Biol. Chem. 286, 22924–22933 (2011).
Moreno, R., Sobotzik, J. M., Schultz, C. & Schmitz, M. L. Specification of the NF-κB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKK ɛ. Nucleic Acids Res. 38, 6029–6044 (2010).
O'Shea, J. M. & Perkins, N. D. Thr435 phosphorylation regulates RelA (p65) NF-κB subunit transactivation. Biochem. J. 426, 345–354 (2010). Reference 57 showed that Ser536- and Ser468-phosphorylated RELA can exist as distinct isoforms within the cell, with different spatial localization and gene-specific regulation of transcription. Reference 58 reached a similar conclusion concerning Thr435 phosphorylation of RELA.
Msaki, A. et al. The role of RelA (p65) threonine 505 phosphorylation in the regulation of cell growth, survival, and migration. Mol. Biol. Cell 22, 3032–3040 (2011).
Anrather, J., Racchumi, G. & Iadecola, C. cis-acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-κB. J. Biol. Chem. 280, 244–252 (2005).
Barre, B. & Perkins, N. D. The Skp2 promoter integrates signaling through the NF-κB, p53, and Akt/GSK3β pathways to regulate autophagy and apoptosis. Mol. Cell 38, 524–538 (2010).
Geng, H., Wittwer, T., Dittrich-Breiholz, O., Kracht, M. & Schmitz, M. L. Phosphorylation of NF-κB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 10, 381–386 (2009).
Mao, X. et al. GCN5 is a required cofactor for a ubiquitin ligase that targets NF-κB/RelA. Genes Dev. 23, 849–861 (2009).
Gapuzan, M. E., Pitoc, G. A. & Gilmore, T. D. Mutations within a conserved protein kinase A recognition sequence confer temperature-sensitive and partially defective activities onto mouse c-Rel. Biochem. Biophys. Res. Commun. 307, 92–99 (2003).
Lawrence, T., Bebien, M., Liu, G. Y., Nizet, V. & Karin, M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434, 1138–1143 (2005).
Bohuslav, J., Chen, L. F., Kwon, H., Mu, Y. & Greene, W. C. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 279, 26115–26125 (2004).
Campbell, K. J., Witty, J. M., Rocha, S. & Perkins, N. D. Cisplatin mimics ARF tumor suppressor regulation of RelA (p65) nuclear factor-κB transactivation. Cancer Res. 66, 929–935 (2006).
Dong, J., Jimi, E., Zhong, H., Hayden, M. S. & Ghosh, S. Repression of gene expression by unphosphorylated NF-κB p65 through epigenetic mechanisms. Genes Dev. 22, 1159–1173 (2008).
Culver, C. et al. Mechanism of hypoxia-induced NF-κB. Mol. Cell. Biol. 30, 4901–4921 (2010).
Hanson, J. L., Hawke, N. A., Kashatus, D. & Baldwin, A. S. The nuclear factor κB subunits RelA/p65 and c-Rel potentiate but are not required for Ras-induced cellular transformation. Cancer Res. 64, 7248–7255 (2004).
Dey, A., Tergaonkar, V. & Lane, D. P. Double-edged swords as cancer therapeutics: simultaneously targeting p53 and NF-κB pathways. Nature Rev. Drug Discov. 7, 1031–1040 (2008).
Ak, P. & Levine, A. J. p53 and NF-κB: different strategies for responding to stress lead to a functional antagonism. FASEB J. 24, 3643–3652 (2010).
Schneider, G. & Kramer, O. H. NFκB/p53 crosstalk-a promising new therapeutic target. Biochim. Biophys. Acta 1815, 90–103 (2011).
Ravi, R. et al. p53-mediated repression of nuclear factor-κB RelA via the transcriptional integrator p300. Cancer Res. 58, 4531–4536 (1998).
Wadgaonkar, R. et al. CREB-binding protein is a nuclear integrator of nuclear factor-κB and p53 signaling. J. Biol. Chem. 274, 1879–1882 (1999).
Webster, G. A. & Perkins, N. D. Transcriptional cross talk between NF-κB and p53. Molecular and cellular biology 19, 3485–3495 (1999).
Huang, W. C., Ju, T. K., Hung., M. C. & Chen, C. C. Phosphorylation of CBP by IKKα promotes cell growth by switching the binding preference of CBP from p53 to NF-κB. Mol. Cell 26, 75–87 (2007).
Tergaonkar, V., Pando, M., Vafa, O., Wahl, G. & Verma, I. p53 stabilization is decreased upon NFκB activation: a role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell 1, 493–503 (2002).
Rocha, S., Campbell, K. J. & Perkins, N. D. p53- and Mdm2-independent repression of NF-κB transactivation by the ARF tumor suppressor. Mol. Cell 12, 15–25 (2003).
Wolff, B. & Naumann, M. INK4 cell cycle inhibitors direct transcriptional inactivation of NF-κB. Oncogene 18, 2663–2666 (1999).
Wang, J., An, H., Mayo, M. W., Baldwin, A. S. & Yarbrough, W. G. LZAP, a putative tumor suppressor, selectively inhibits NF-κB. Cancer Cell 12, 239–251 (2007).
Kashima, L. et al. CHFR, a potential tumor suppressor, downregulates interleukin-8 through the inhibition of NF-κB. Oncogene 28, 2643–2653 (2009).
Rattan, R. et al. TCEAL7, a putative tumor suppressor gene, negatively regulates NF-κB pathway. Oncogene 29, 1362–1373 (2010).
Mayo, M. W. et al. PTEN blocks tumor necrosis factor-induced NF-κB-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J. Biol. Chem. 277, 11116–11125 (2002).
Mauro, C. et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nature Cell Biol. 13, 1272–1279 (2011). This paper reveals that RELA-dependent upregulation of p53 levels results in increased oxidative phosphorylation and indicates that this is a new mechanism through which NF-κB can function as a tumour suppressor.
Shetty, S. et al. Transcription factor NF-κB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol. Cell. Biol. 25, 5404–5416 (2005).
Frank, A. K. et al. The codon 72 polymorphism of p53 regulates interaction with NF-κB and transactivation of genes involved in immunity and inflammation. Mol. Cell Biol. 31, 1201–1213 (2011). This paper confirms, in a humanized p53 mouse model, that NF-κB and p53 can function co-operatively rather than antagonistically in some contexts. The extent of this effect seems to be at least partly dependent on a common polymorphism present in p53. This also highlights a potential difference in the relationship between p53 and NF-κB in mice and humans.
Martin, A. G., Trama, J., Crighton, D., Ryan, K. M. & Fearnhead, H. O. Activation of p73 and induction of Noxa by DNA damage requires NF-κB. Aging 1, 335–349 (2009).
O'Prey, J. et al. p53-mediated induction of Noxa and p53AIP1 requires NFκB. Cell Cycle 9, 947–952 (2010).
Schumm, K., Rocha, S., Caamano, J. & Perkins, N. D. Regulation of p53 tumour suppressor target gene expression by the p52 NF-κB subunit. EMBO J. 25, 4820–4832 (2006).
Schneider, G. et al. Cross talk between stimulated NF-κB and the tumor suppressor p53. Oncogene 29, 2795–2806 (2010).
Choy, M. K. et al. High-throughput sequencing identifies STAT3 as the DNA-associated factor for p53-NF-κB-complex-dependent gene expression in human heart failure. Genome Med. 2, 37 (2010).
Lu, H. et al. TNF-α promotes c-REL/ΔNp63α interaction and TAp73 dissociation from key genes that mediate growth arrest and apoptosis in head and neck cancer. Cancer Res. 71, 6867–6877 (2011).
Dell'Orso, S. et al. ChIP-on-chip analysis of in vivo mutant p53 binding to selected gene promoters. OMICS 15, 305–312 (2011).
Weisz, L. et al. Mutant p53 enhances nuclear factor κB activation by tumor necrosis factor α in cancer cells. Cancer Res. 67, 2396–2401 (2007).
Kawahara, T. L. et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136, 62–74 (2009).
Orjalo, A. V., Bhaumik, D., Gengler, B. K., Scott, G. K. & Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl Acad. Sci. USA 106, 17031–17036 (2009).
Wang, J. et al. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO Rep. 10, 1272–1278 (2009). This paper demonstrates that loss of RELA results in genomic instability and reduced DNA repair after damage, adding to the number of potentially tumour-suppressing characteristics of NF-κB.
Rovillain, E. et al. Activation of nuclear factor-κB signalling promotes cellular senescence. Oncogene 30, 2356–2366 (2011).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Jing, H. et al. Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev. 25, 2137–2146 (2011). References 100 and 101 both identify tumour suppressor-like characteristics of RELA and NF-κB associated with the induction of senescence.
Ghiorzo, P. et al. Inverse correlation between p16INK14A expression and NF-κB activation in melanoma progression. Hum. Pathol. 35, 1029–1037 (2004).
Lee, T. L. et al. A novel nuclear factor-κB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clin. Cancer Res. 13, 5680–5691 (2007).
Adli, M. & Baldwin, A. S. IKK-i/IKKɛ controls constitutive, cancer cell-associated NF-κB activity via regulation of Ser-536 p65/RelA phosphorylation. J. Biol. Chem. 281, 26976–26984 (2006).
Dan, H. C. et al. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 22, 1490–1500 (2008).
Madrid, L. V. et al. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20, 1626–1638, (2000).
Renner, F., Moreno, R. & Schmitz, M. L. SUMOylation-dependent localization of IKKɛ in PML nuclear bodies is essential for protection against DNA-damage-triggered cell death. Mol. Cell 37, 503–515 (2010).
Beg, A. A., Sha, W. C., Bronson, R. T., Ghosh, S. & Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376, 167–170 (1995).
Wu, Z. H. & Miyamoto, S. Many faces of NF-κB signaling induced by genotoxic stress. J. Mol. Med. 85, 1187–1202 (2007).
Stilmann, M. et al. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IκB kinase activation. Mol. Cell 36, 365–378 (2009).
Wu, Z. H. et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol. Cell 40, 75–86 (2010).
Kenneth, N. S., Mudie, S. & Rocha, S. IKK and NF-κB-mediated regulation of Claspin impacts on ATR checkpoint function. EMBO J. 29, 2966–2978 (2010). This paper reveals the potential for NF-κB to influence the checkpoint kinase response to DNA damage by demonstrating that claspin, an important regulator of CHK1 activity, is a REL target.
Wu, Z. H. & Miyamoto, S. Induction of a pro-apoptotic ATM-NF-κB pathway and its repression by ATR in response to replication stress. EMBO J. 27, 1963–1973 (2008). This paper shows that DNA damage associated with replication stress results in pro-apoptotic functions of NF-κB. References 112 and 113 further illustrate that the consequences of the NF-κB response to DNA damage differ from that seen with inflammatory cytokines.
Ho, J. Q., Asagiri, M., Hoffmann, A. & Ghosh, G. NF-κB potentiates caspase independent hydrogen peroxide induced cell death. PLoS ONE 6, e16815 (2011).
Barre, B., Coqueret, O. & Perkins, N. D. Regulation of activity and function of the p52 NF-κB subunit following DNA damage. Cell Cycle 9, 4795–4804 (2010).
Bednarski, B. K., Baldwin, A. S. Jr & Kim, H. J. Addressing reported pro-apoptotic functions of NF-κB: targeted inhibition of canonical NF-κB enhances the apoptotic effects of doxorubicin. PLoS ONE 4, e6992 (2009).
Karl, S. et al. Identification of a novel pro-apopotic function of NF-κB in the DNA damage response. J. Cell. Mol. Med. 13, 4239–4256 (2009).
Bian, X. et al. NF-κB activation mediates doxorubicin-induced cell death in N-type neuroblastoma cells. J. Biol. Chem. 276, 48921–48929 (2001).
Campbell, K. J., O'Shea, J. M. & Perkins, N. D. Differential regulation of NF-κB activation and function by topoisomerase II inhibitors. BMC Cancer 6, 101 (2006).
Ho, W. C., Dickson, K. M. & Barker, P. A. Nuclear factor-κB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-κB-dependent transcription in cancer cells. Cancer Res. 65, 4273–4281 (2005).
Manna, S. K., Manna, P. & Sarkar, A. Inhibition of RelA phosphorylation sensitizes apoptosis in constitutive NF-κB-expressing and chemoresistant cells. Cell Death Differ. 14, 158–170 (2007).
Rocha, S., Garrett, M. D., Campbell, K. J., Schumm, K. & Perkins, N. D. Regulation of NF-κB and p53 through activation of ATR and Chk1 by the ARF tumour suppressor. EMBO J. 24, 1157–1169 (2005).
Roccaro, A. M., Vacca, A. & Ribatti, D. Bortezomib in the treatment of cancer. Recent Pat. Anticancer Drug Discov. 1, 397–403 (2006).
Karin, M., Yamamoto, Y. & Wang, Q. M. The IKK NF-κB system: a treasure trove for drug development. Nature Rev. Drug Discov. 3, 17–26 (2004).
Gilmore, T. D. & Garbati, M. R. Inhibition of NF-κB signaling as a strategy in disease therapy. Curr. Top. Microbiol. Immunol. 349, 245–263 (2011).
Avila, C. M. et al. Structure-based design and biological profile of (E)-N-(4-Nitrobenzylidene)-2-naphthohydrazide, a novel small molecule inhibitor of IκB kinase-β. Eur. J. Med. Chem. 46, 1245–1253 (2011).
Chiang, P. C., Kishore, N. N. & Thompson, D. C. Combined use of pharmacokinetic modeling and a steady-state delivery approach allows early assessment of IκB kinase-2 (IKK-2) target safety and efficacy. J. Pharm. Sci. 99, 1278–1287 (2010).
Kim, S. et al. Discovery of piperidinyl aminopyrimidine derivatives as IKK-2 inhibitors. Bioorg Med. Chem. Lett. 21, 3002–3006 (2011).
Mbalaviele, G. et al. A novel, highly selective, tight binding IκB kinase-2 (IKK-2) inhibitor: a tool to correlate IKK-2 activity to the fate and functions of the components of the nuclear factor-κB pathway in arthritis-relevant cells and animal models. J. Pharmacol. Exp. Ther. 329, 14–25 (2009).
Kwak, J. H., Jung, J. K. & Lee, H. Nuclear factor-κB inhibitors; a patent review (2006 - 2010). Expert Opin. Ther. Pat. 21, 1897–1910 (2011).
Greten, F. R. et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130, 918–931 (2007).
Pasparakis, M. Regulation of tissue homeostasis by NF-κB signalling: implications for inflammatory diseases. Nature Rev. Immunol. 9, 778–788 (2009).
Oh, U. et al. Inhibition of immune activation by a novel nuclear factor-κB inhibitor in HTLV-I-associated neurologic disease. Blood 117, 3363–3369 (2011).
Shangary, S. & Wang, S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu. Rev. Pharmacol. Toxicol. 49, 223–241 (2009).
Zheng, C., Yin, Q. & Wu, H. Structural studies of NF-κB signaling. Cell Res. 21, 183–195 (2011).
Takada, Y., Singh, S. & Aggarwal, B. B. Identification of a p65 peptide that selectively inhibits NF-κB activation induced by various inflammatory stimuli and its role in down-regulation of NF-κB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 279, 15096–15104 (2004).
Oakley, F. et al. Angiotensin II activates I κB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 136, 2334–2344.e1 (2009).
Buss, H. et al. Phosphorylation of serine 468 by GSK-3β negatively regulates basal p65 NF-κB activity. J. Biol. Chem. 279, 49571–49574 (2004).
Barre, B. & Perkins, N. D. A cell cycle regulatory network controlling NF-κB subunit activity and function. EMBO J. 26, 4841–4855 (2007).
Rocha, S., Martin, A. M., Meek, D. W. & Perkins, N. D. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-κB subunit with histone deacetylase 1. Mol. Cell. Biol. 23, 4713–4727 (2003).
Westerheide, S. D., Mayo, M. W., Anest, V., Hanson, J. L. & Baldwin, A. S. Jr. The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition. Mol. Cell. Biol. 21, 8428–8436 (2001).
Guma, M. et al. Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 208, 1889–1900 (2011).
Dey, A., Wong, E. T., Bist, P., Tergaonkar, V. & Lane, D. P. Nutlin-3 inhibits the NFκB pathway in a p53-dependent manner: implications in lung cancer therapy. Cell Cycle 6, 2178–2185 (2007).
Calvert, H. & Azzariti, A. The clinical development of inhibitors of poly(ADP-ribose) polymerase. Ann. Oncol. 22, i53–i59 (2011).
Hunter, J. E. et al. NF-κB mediates radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene, 27 Jun 2011 (doi:10.1038/onc.2011.229).
Veuger, S. J., Hunter, J. E. & Durkacz, B. W. Ionizing radiation-induced NF-κB activation requires PARP-1 function to confer radioresistance. Oncogene 28, 832–842 (2009).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature, 478 524–528 (2011).
Lee, S. T. et al. Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol. Cell 43, 798–810 (2011).
van Essen, D., Zhu, Y. & Saccani, S. A feed-forward circuit controlling inducible NF-κB target gene activation by promoter histone demethylation. Mol. Cell 39, 750–760 (2010). This report provides important insights into the mechanism of how REL regulates target gene transcription, and also shows how such studies may lead to the identification of druggable proteins with which to target NF-κB activity.
Teo, H. et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nature Cell Biol. 12, 758–767 (2010). This paper shows that phosphorylation of Ser536 is carried out by a distinct IKK complex containing RAP1, a protein previously associated with telomere regulation. This suggests that it may be possible to therapeutically target specific activities of the IKK complex without global inhibition of this signalling pathway.
Chew, J. et al. WIP1 phosphatase is a negative regulator of NF-κB signalling. Nature Cell Biol. 11, 659–666 (2009). This paper reports that dephosphorylation of Ser536 is an important regulatory mechanism, with implications for loss of control of NF-κB signalling in inflammatory diseases and cancer.
Kato, T., Delhase, M., Hoffmann, A. & Karin, M. CK2 is a C-terminal I κB kinase responsible for NF-κB activation during the UV response. Mol. Cell 12, 829–839 (2003).
Matsuzawa, A. et al. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science 321, 663–668 (2008).
Bettermann, K. et al. TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell 17, 481–496 (2010).
De Santa, F. et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 28, 3341–3352 (2009).
Trecca, D. et al. Identification of a tumor-associated mutant form of the NF- κB RelA gene with reduced DNA-binding and transactivating activities. Oncogene 14, 791–799 (1997).
Starczynowski, D. T. et al. Mutation of an IKK phosphorylation site within the transactivation domain of REL in two patients with B-cell lymphoma enhances REL's in vitro transforming activity. Oncogene 26, 2685–2694 (2007). This paper provides one of the few examples of a tumour-associated NF-κB subunit mutation with a defined effect on function and transformation. An important question is why more similar mutations have not been described.
Hashimoto, R. et al. Variants of the RELA gene are associated with schizophrenia and their startle responses. Neuropsychopharmacology 36, 1921–1931 (2011).
Tan, B. H., Ross, J. A., Kaasa, S., Skorpen, F. & Fearon, K. C. Identification of possible genetic polymorphisms involved in cancer cachexia: a systematic review. J. Genet. 90, 165–177 (2011).
Zou, Y. F. et al. Association between NFKB1 -94ins/delATTG promoter polymorphism and cancer risk: a meta-analysis. Cancer Invest. 29, 78–85 (2011).
Forbes, S. A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945–D950 (2011).
Acknowledgements
The author would like to thank D. Mann, S. Rocha, K. Campbell and all members of the N.D.P. laboratory for their critical reading of this manuscript, together with T. Gilmore, V. Tergaonkar and M. Lienhard Schmitz for helpful discussions. Research in the Perkins' laboratory is funded by Cancer Research UK (grants C1443/A12750 and C1443/A6721), the Wellcome Trust (grant 094,409), European Union FP7 'Inflacare' consortium and Leukemia and Lymphoma Research (grant 11022). The author would like to apologize to all colleagues whose work he was unable to cite in this Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- MicroRNAs
-
These single-stranded RNAs are approximately 21 to 23 nucleotides in length and regulate gene expression by partial complementary base pairing to mRNAs and recruitment to the RNA-induced silencing complex to inhibit translation (and possibly increase degradation) of mRNA.
- Warburg effect
-
Named after a discovery made by the German biochemist Otto Warburg in the 1920s that cancer cells predominantly use anaerobic glycolysis rather than oxidative phosphorylation, even when oxygen is abundant. As a result, pyruvate is converted to lactate instead of being oxidized by the mitochondria of cancer cells.
- Isogenically matched
-
Genetically identical.
Rights and permissions
About this article
Cite this article
Perkins, N. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer 12, 121–132 (2012). https://doi.org/10.1038/nrc3204
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3204
This article is cited by
-
Drug repurposing for cancer therapy
Signal Transduction and Targeted Therapy (2024)
-
The linkage of NF-κB signaling pathway-associated long non-coding RNAs with tumor microenvironment and prognosis in cervical cancer
BMC Medical Genomics (2023)
-
UBE2J1 inhibits colorectal cancer progression by promoting ubiquitination and degradation of RPS3
Oncogene (2023)
-
Polo-like kinase 4 (Plk4) potentiates anoikis-resistance of p53KO mammary epithelial cells by inducing a hybrid EMT phenotype
Cell Death & Disease (2023)
-
Sodium selenite inhibits proliferation and metastasis through ROS‐mediated NF‐κB signaling in renal cell carcinoma
BMC Cancer (2022)