Key Points
-
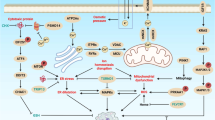
A number of alternative cell death programmes, such as necroptosis, lysosomal-mediated programmed cell death (LM-PCD) and autophagy have been established alongside classical apoptosis. These are now known to act both as a backup to apoptosis and also as preferred death pathways in certain cell types.
-
Necroptosis can be triggered by a RIP1- and RIP3-containing complex. This complex can form downstream of death receptors or in the cytosol following stress stimuli. It is tightly regulated by the initiator caspase 8, as well as inhibitors of apoptosis (IAPs) and FLICE-like inhibitory protein (FLIP). Hence, caspase inhibitors, as well as second mitochondria-derived activator of caspases (SMAC) mimetics, are strong sensitizers for necroptosis.
-
LM-PCD, which occurs because of a loss of lysosomal integrity, is frequently seen in cancer cells. This is due to increased metabolism and protein turnover, as well as a reduction of important lysosomal structural proteins. Cancer cells are thus particularly responsive to drugs that target the lysosomes, and drugs ranging from lysosomotropic agents to monoclonal antibodies and microtubule-disrupting agents have all been shown to induce LM-PCD.
-
Autophagy can have both tumour suppressive and tumour-promoting activities. Large-scale autophagy can eventually lead to cell death; however, it is not clear precisely how this type of death is induced. Inhibition and induction of autophagy have both proved to be beneficial under certain circumstances, and the decision as to whether inhibition or activation is preferable is still largely empirical.
-
The alternative pathways of cell death prove to be intricately interconnected with many points of convergence, such as JUN N-terminal kinase (JNK), AMP-activated protein kinase (AMPK) or reactive oxygen species (ROS). Necroptosis has LM-PCD as a frequent downstream occurrence, and an impaired lysosomal compartment affects the ability of autophagosomes to mature. These converging and diverging features are increasingly better understood, leading to a number of targeted approaches aimed at these alternative pathways.
Abstract
Evading programmed cell death is one of the hallmarks of cancer. Conversely, inducing cell death by pharmacological means is the basis of almost every non-invasive cancer therapy. Research over the past decade has greatly increased our understanding of non-apoptotic programmed cell death events, such as lysosomal-mediated cell death, necroptosis and cell death with autophagy. It is becoming clear that an intricate effector network connects many of these classical and non-classical death pathways. In this Review, we discuss converging and diverging features of these pathways, as well as attempts to exploit this newly gained knowledge pharmacologically to provide therapeutics for cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Ellis, H. M. & Horvitz, H. R. Genetic control of programmed cell death in the nematode, C. elegans. Cell 44, 817–829 (1986).
Earnshaw, W. C., Martins, L. M. & Kaufmann, S. H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68, 383–424 (1999).
Lavrik, I., Golks, A. & Krammer, P. H. Death receptor signaling. J. Cell Sci. 118, 265–267 (2005).
Itoh, N. et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66, 233–243 (1991).
Chipuk, J. E., Moldoveanu, T., Llambi, F., Parsons, M. J. & Green, D. R. The BCL-2 family reunion. Mol. Cell 37, 299–310 (2010).
Tsujimoto, Y., Cossman, J., Jaffe, E. & Croce, C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science 228, 1440–1443 (1985).
Galluzzi, L. et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19, 107–120 (2012).
Kroemer, G. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 (2009).
Kreuzaler, P. A. et al. Stat3 controls lysosomal-mediated cell death in vivo. Nature Cell Biol. 13, 303–309 (2011). This study demonstrated for the first time that a lysosomal-mediated pathway of cell death is the principal pathway in a physiological cell death event. It also indicated that cells can withstand widespread LMP, if protective factors to inhibit cathepsin activity are upregulated in the cytosol.
Maiuri, M. C., Zalckvar, E., Kimchi, A. & Kroemer, G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Rev. Mol. Cell Biol. 8, 741–752 (2007).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chem. Biol. 1, 112–119 (2005). This paper identifies the RIP1 inhibitor necrostatin 1 (NEC1) as a potent inhibitor of necroptosis and also demonstrates its efficacy in delaying ischaemic brain injury in mice. It formed the basis of many studies using NEC1 as a tool to study necroptosis.
Weinlich, R., Dillon, C. P. & Green, D. R. Ripped to death. Trends Cell Biol. 21, 630–637 (2011).
Boya, P. & Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 27, 6434–6451 (2008).
Vitale, I., Galluzzi, L., Castedo, M. & Kroemer, G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nature Rev. Mol. Cell Biol. 12, 385–392 (2011).
Overholtzer, M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007).
Kepp, O. et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Met. Rev. 30, 61–69 (2011).
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immun. 141, 2629–2634 (1988).
Lemaire, C., Andréau, K., Souvannavong, V. & Adam, A. Inhibition of caspase activity induces a switch from apoptosis to necrosis. FEBS Lett. 425, 266–270 (1998).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998). This is one of the first studies showing a cytoprotective effect of caspases downstream of TNFR. It also establishes ROS as a mediator for necrosis following concomitant TNFα stimulation and caspase inhibition.
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 1, 489–495 (2000).
Vanlangenakker, N., Vanden Berghe, T. & Vandenabeele, P. Many stimuli pull the necrotic trigger, an overview. Cell Death Diff. 19, 75–86 (2011).
Varfolomeev, E. E. et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Hsu, H., Huang, J., Shu, H. B., Baichwal, V. & Goeddel, D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Ea, C.-K., Deng, L., Xia, Z.-P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Cho, Y. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009).
Zhang, D.-W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009). This study showed that RIP3 is an obligate partner of RIP1-induced necroptosis. More importantly, it revealed that RIP3 recruits and activates many metabolic enzymes, thus coordinating some of the metabolic shifts that are thought to drive necroptosis.
Kuida, K. et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384, 368–372 (1996).
Kuida, K. et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94, 325–337 (1998).
Hakem, R. et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94, 339–352 (1998).
Zhang, J., Cado, D., Chen, A., Kabra, N. H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 (1998).
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Yeh, W. C. et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12, 633–642 (2000).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Zhang, H. et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 (2011).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011). References 36, 37 and 38 identified the cytoprotective role of caspase 8 on the RIP1–RIP3 complex and explained why the knockout of caspase 8 or FADD was embryonic lethal. Reference 36 also showed that a caspase 8–FLIPL complex retains sufficient activity to suppress necrosis, but too little to induce apoptosis.
O'Donnell, M. A. et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature Cell Biol. 13, 1437–1442 (2011).
Tenev, T. et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 (2011). Along with reference 83, this study established the ripoptosome as a death receptor-independent cell death signalling platform that forms after genotoxic stress or SMAC-mimetic treatment.
Leist, M., Single, B., Castoldi, A. F., Kühnle, S. & Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185, 1481–1486 (1997).
Schulze-Osthoff, K. et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267, 5317–5323 (1992).
Suffys, P. et al. Tumour-necrosis-factor-mediated cytotoxicity is correlated with phospholipase-A2 activity, but not with arachidonic acid release per se. Eur. J. Biochem. 195, 465–475 (1991).
Edinger, A. L. & Thompson, C. B. Death by design: apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 16, 663–669 (2004).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Rev. Mol. Cell Biol. 11, 700–714 (2010).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). Through a large compound screen, this paper identified MLKL as an obligate member of the RIP1–RIP3 complex, leading to induction of necroptosis. MLKL is phosphorylated by RIP3 and its absence blocks necroptosis after formation of the necroptosome.
Guicciardi, M. E., Leist, M. & Gores, G. J. Lysosomes in cell death. Oncogene 23, 2881–2890 (2004).
Ferri, K. F. & Kroemer, G. Organelle-specific initiation of cell death pathways. Nature Cell Biol. 3, E255–E263 (2001).
Foghsgaard, L. et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153, 999–1010 (2001).
Liu, N. et al. NF-kappaB protects from the lysosomal pathway of cell death. EMBO J. 22, 5313–5322 (2003).
Groth-Pedersen, L. & Jäättelä, M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 30 Jun 2010 (doi:10.1016/j.canlet.2010.05.021).
Jäättelä, M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene 23, 2746–2756 (2004).
Turk, B. et al. Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biol. Chem. 376, 225–230 (1995).
Droga-Mazovec, G. et al. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 283, 19140–19150 (2008).
Hishita, T. et al. Caspase-3 activation by lysosomal enzymes in cytochrome c-independent apoptosis in myelodysplastic syndrome-derived cell line P39. Cancer Res. 61, 2878–2884 (2001).
Roberts, L. R. et al. Cathepsin B contributes to bile salt-induced apoptosis of rat hepatocytes. Gastroenterology 113, 1714–1726 (1997).
Vancompernolle, K. et al. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 438, 150–158 (1998).
Jäättelä, M. & Tschopp, J. Caspase-independent cell death in T lymphocytes. Nature Immunol. 4, 416–423 (2003).
Turk, V. et al. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophhys. Acta 1824, 68–88 (2012).
Liu, N. et al. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nature Immunol. 5, 919–926 (2004).
Klionsky, D. J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nature Rev. Mol. Cell Biol. 8, 931–937 (2007).
Kroemer, G., Mariño, G. & Levine, B. Autophagy and the integrated stress response. Molecular Cell 40, 280–293 (2010).
Lee, J. W., Park, S., Takahashi, Y. & Wang, H.-G. The association of AMPK with ULK1 regulates autophagy. PloS ONE 5, e15394 (2010).
Kim, J., Kundu, M., Viollet, B. & Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biol. 13, 132–141 (2011).
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005). Using Bax−/−; Bak−/− cells that are resistant to apoptosis, this study established the importance of autophagy in sustaining cellular metabolism in growth factor-deprived cells. Cells remained viable for weeks and responded to growth factors when these were supplemented.
Baehrecke, E. H. Autophagic programmed cell death in Drosophila. Cell Death Diff. 10, 940–945 (2003).
Tresse, E., Kosta, A., Luciani, M.-F. & Golstein, P. From autophagic to necrotic cell death in Dictyostelium. Semin. Cancer Biol. 17, 94–100 (2007).
Shimizu, S. et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cell Biol. 6, 1221–1228 (2004).
Yu, L. et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304, 1500–1502 (2004).
Eskelinen, E.-L. The dual role of autophagy in cancer. Curr. Opin. Pharmacol. 11, 294–300 (2011).
Notte, A., Leclere, L. & Michiels, C. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem. Pharmacol. 82, 427–434 (2011).
Lee, J.-S. et al. FLIP-mediated autophagy regulation in cell death control. Nature Cell Biol. 11, 1355–1362 (2009).
Maiuri, M. C. et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26, 2527–2539 (2007).
Wirawan, E. et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 1, e18 (2010).
Yousefi, S. et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nature Cell Biol. 8, 1124–1132 (2006). This paper showed that overexpression of ATG5 sensitizes cells to apoptotic stimuli. This is mediated by the cleavage of ATG5 by calpains and subsequent concomitant translocation of the N-terminal product and BCL-X L to the mitochondrion, where cytochrome c is released.
Giansanti, V., Torriglia, A. & Scovassia, I. Conversation between apoptosis and autophagy: “Is it your turn or mine?”. Apoptosis 16, 321–333 (2011).
Yu, L. et al. Autophagic programmed cell death by selective catalase degradation. Proc. Natl Acad. Sci. 103, 4952–4957 (2006). This study showed that in some circumstances attenuation of autophagy can reduce accumulation of ROS. This is due to the autophagic degradation of the enzymatic ROS scavenger catalase, which is initiated by caspase inhibition.
Brosh, R. & Rotter, V. When mutants gain new powers: news from the mutant p53 field. Nature Rev. Cancer 9, 701–713 (2009).
Kelly, P. N. & Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Diff. 18, 1414–1424 (2011).
Coupienne, I., Fettweis, G. & Piette, J. RIP3 expression induces a death profile change in U2OS osteosarcoma cells after 5-ALA-PDT. Las. Surg. Med. 43, 557–564 (2011).
Mantel, F. et al. Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenth. Onkol. 186, 587–599 (2010).
Nehs, M. A. et al.Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery 150, 1032–1039 (2011).
Feoktistova, M. et al. cIAPs block ripoptosome formation, a RIP1/Caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011). This publication, along with Reference 40, studied the formation and regulation of the ripoptosome. It has a special focus on the influence of the ripoptosome on TLR3.
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Krueger, A., Schmitz, I., Baumann, S., Krammer, P. H. & Kirchhoff, S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276, 20633–20640 (2001).
Bertrand, M. J. M. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008). This study established IAP1 and IAP2 as E3 ligases that ubiquitylate RIP1 in cancer cells. This impedes cell death and inhibition of IAPs with the compound AEG40730 restores death induction.
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Chai, J. et al. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406, 855–862 (2000).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131, 682–693 (2007).
Darding, M. & Meier, P. IAPs: Guardians of RIPK1. Cell Death Differ. 19, 58–66 (2011).
Vanlangenakker, N., Bertrand, M. J. M., Bogaert, P., Vandenabeele, P. & Vanden Berghe, T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2, e230 (2011).
Sloane, B. F., Dunn, J. R. & Honn, K. V. Lysosomal cathepsin B: correlation with metastatic potential. Science 212, 1151–1153 (1981).
Joyce, J. A. et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 5, 443–453 (2004).
Vasiljeva, O. et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 66, 5242–5250 (2006).
Sevenich, L. et al. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene 30, 54–64 (2011).
Leto, G. et al. Lysosomal cathepsins B and L and Stefin A blood levels in patients with hepatocellular carcinoma and/or liver cirrhosis: potential clinical implications. Oncology 54, 79–83 (1997).
Mikhaylov, G. et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nature Nanotech. 6, 594–602 (2011).
Sevenich, L. et al. Synergistic antitumor effects of combined cathepsin B and cathepsin Z. deficiencies on breast cancer progression and metastasis in mice. Proc. Natl Acad. Sci. USA 107, 2497–2502 (2010).
Reeves, J. P. Accumulation of amino acids by lysosomes incubated with amino acid methyl esters. J. Biol. Chem. 254, 8914–8921 (1979).
Kirkegaard, T. & Jäättelä, M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta 1793, 746–754 (2009).
Fehrenbacher, N. et al. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res. 68, 6623–6633 (2008).
Fehrenbacher, N. et al. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 64, 5301–5310 (2004).
Kagedal, K., Zhao, M., Svensson, I., Brunk, U. T. & Kågedal, K. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359, 335–343 (2001).
Rammer, P. et al. BAMLET activates a lysosomal cell death program in cancer cells. Molecular Cancer Therap. 9, 24–32 (2010).
Boya, P. et al. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22, 3927–3936 (2003).
Ostenfeld, M. S. et al. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 65, 8975–8983 (2005).
Terman, A., Kurz, T., Gustafsson, B. & Brunk, U. T. Lysosomal labilization. IUBMB Life 58, 531–539 (2006).
Puissant, A. et al. Cathepsin B release after imatinib-mediated lysosomal membrane permeabilization triggers BCR-ABL cleavage and elimination of chronic myelogenous leukemia cells. Leukemia 24, 115–124 (2010). This paper showed that the BCR–ABL inhibitor imatinib not only acts directly on BCR-ABL, but also induces an amplifying loop in which lysosomes become leaky and cathepsin B degrades the oncogenic protein BCR-ABL itself.
Alduaij, W. et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117, 4519–4529 (2011).
Bröker, L. E. et al. Cathepsin B mediates caspase-independent cell death induced by microtubule stabilizing agents in non-small cell lung cancer cells. Cancer Res. 64, 27–30 (2004).
Groth-Pedersen, L., Ostenfeld, M. S., Høyer-Hansen, M., Nylandsted, J. & Jäättelä, M. Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res. 67, 2217–2225 (2007).
Zamora, J. M. & Beck, W. T. Chloroquine enhancement of anticancer drug cytotoxicity in multiple drug resistant human leukemic cells. Biochem. Pharmacol. 35, 4303–4310 (1986).
De Milito, A. et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 67, 5408–5417 (2007).
Mak, I. T. & Weglicki, W. B. Characterization of iron-mediated peroxidative injury in isolated hepatic lysosomes. J. Clin. Invest 75, 58–63 (1985).
Baird, S. K., Kurz, T. & Brunk, U. T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem. J. 394, 275–283 (2006).
Persson, H. L., Yu, Z., Tirosh, O., Eaton, J. W. & Brunk, U. T. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Rad. Biol. Med. 34, 1295–1305 (2003).
Calderwood, S. K., Khaleque, M. A., Sawyer, D. B. & Ciocca, D. R. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 31, 164–172 (2006).
Leu, J. I.-J., Pimkina, J., Frank, A., Murphy, M. E. & George, D. L. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 36, 15–27 (2009). In this study, the authors established a chemical inhibitor (PES) for HSP70. They showed that it induces caspase-independent cell death, which is accompanied by impaired lysosomal function and accumulation of large vacuoles.
Kirkegaard, T. et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463, 549–553 (2010). This study showed that HSP70 stabilizes lysosomes by activating the acid sphingomyelinase ( ASM ). This rationale was used to block cell death in cells from patients with Nieman–Pick disease.
Mohamed, M. M. & Sloane, B. F. Cysteine cathepsins: multifunctional enzymes in cancer. Nature Rev. Cancer 6, 764–775 (2006).
Appelqvist, H. et al. Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am. J. Pathol. 178, 629–639 (2011).
Sehested, M., Skovsgaard, T., van Deurs, B. & Winther-Nielsen, H. Increased plasma membrane traffic in daunorubicin resistant P388 leukaemic cells. Effect of daunorubicin and verapamil. Br. J. Cancer 56, 747–751 (1987).
Shiraishi, N., Akiyama, S., Kobayashi, M. & Kuwano, M. Lysosomotropic agents reverse multiple drug resistance in human cancer cells. Cancer Lett. 30, 251–259 (1986).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003).
Huang, X., Bai, H.-M., Chen, L., Li, B. & Lu, Y.-C. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J. Clin. Neuro. 17, 1515–1519 (2010).
Iqbal, J. et al. Genomic analyses reveal global functional alterations that promote tumor growth and novel tumor suppressor genes in natural killer-cell malignancies. Leukemia 23, 1139–1151 (2009).
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Mathew, R. et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 137, 1062–1075 (2009). This study showed that defective autophagy leads to increased levels of ROS and p62. Furthermore, it established that the accumulation of p62 enhances oxidative stress, alters NF-κB signalling and leads to a more tumorigenic gene signature.
Mathew, R. et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 21, 1367–1381 (2007).
Jin, S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2, 80–84 (2006).
Degenhardt, K. et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10, 51–64 (2006).
Koukourakis, M. I. et al. Beclin 1 over- and underexpression in colorectal cancer: distinct patterns relate to prognosis and tumour hypoxia. Br. J. Cancer 103, 1209–1214 (2010).
Fung, C., Lock, R., Gao, S., Salas, E. & Debnath, J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797–806 (2008).
Martinez-Outschoorn, U. E. et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9, 3256–3276 (2010).
Abedin, M. J., Wang, D., McDonnell, M. A., Lehmann, U. & Kelekar, A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 14, 500–510 (2007).
Zhu, K., Dunner, K. & McConkey, D. J. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene 29, 451–462 (2010).
Vazquez-Martin, A., Oliveras-Ferraros, C. & Menendez, J. A. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PloS ONE 4, e6251 (2009).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011). This study showed that oncogenic RAS-driven tumours become addicted to autophagy, which is crucial to maintain a pool of healthy mitochondria. Impairment of autophagy leads to accumulation of abnormal mitochondria and depletion of TCA cycle intermediates.
Elgendy, M., Sheridan, C., Brumatti, G. & Martin, S. J. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol. Cell 42, 23–35 (2011). This study showed that oncogenic RAS can kill cells via an autophagic pathway in culture. Mechanistically, this happens through the upregulation of the death promoting protein NOXA and beclin 1. NOXA can displace MCL1 from beclin 1, thus enhancing autophagy which is followed by cell death.
Nylandsted, J. et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200, 425–435 (2004).
Kim, Y.-S., Morgan, M. J., Choksi, S. & Liu, Z.-G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 26, 675–687 (2007).
Schütze, S., Tchikov, V. & Schneider-Brachert, W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nature Rev. Mol. Cell Biol. 9, 655–662 (2008).
Los, M. et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 13, 978–988 (2002).
Ono, K. et al. Metaxin deficiency alters mitochondrial membrane permeability and leads to resistance to TNF-induced cell killing. Protein Cell 1, 161–173 (2010).
Temkin, V., Huang, Q., Liu, H., Osada, H. & Pope, R. M. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol. Cell. Biol. 26, 2215–2225 (2006).
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biol. 13, 1016–1023 (2011).
Scherz-Shouval, R. & Elazar, Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Scien. 36, 30–38 (2011).
Seglen, P. O. & Gordon, P. B. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl Acad. Sci. USA 79, 1889–1892 (1982).
Powis, G. et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 54, 2419–2423 (1994).
Blommaart, E. F., Krause, U., Schellens, J. P., Vreeling-Sindelárová, H. & Meijer, A. J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. FEBS 243, 240–246 (1997).
Yang, Z. J., Chee, C. E., Huang, S. & Sinicrope, F. a The role of autophagy in cancer: therapeutic implications. Mol. Cancer Therap. 10, 1533–1541 (2011).
Boya, P. et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 25, 1025–1040 (2005).
Yamamoto, A. et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struc. Func. 23, 33–42 (1998).
Kroemer, G. & Jäättelä, M. Lysosomes and autophagy in cell death control. Nature Rev. Cancer 5, 886–897 (2005).
Shingu, T. et al. Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. International journal of cancer. J. Int. Cancer 124, 1060–1071 (2009).
Shen, S. et al. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene 30, 4544–4556 (2011).
Gottlieb, R. A. & Mentzer, R. M. Autophagy during cardiac stress: joys and frustrations of autophagy. Ann. Rev. Phys. 72, 45–59 (2010).
Yang, Y. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874–877 (2000).
LaCasse, E. C. et al. IAP-targeted therapies for cancer. Oncogene 27, 6252–6275 (2008).
Riedl, S. J. & Salvesen, G. S. The apoptosome: signalling platform of cell death. Nature Rev. Mol. Cell Biol. 8, 405–413 (2007).
Malladi, S., Challa-Malladi, M., Fearnhead, H. O. & Bratton, S. B. The Apaf-1 procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer. EMBO J. 28, 1916–1925 (2009).
Mace, P. D. & Riedl, S. J. Molecular cell death platforms and assemblies. Curr. Opin. Cell Biol. 22, 828–836 (2010).
Janssens, S., Tinel, A., Lippens, S. & Tschopp, J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell 123, 1079–1092 (2005).
Katayama, M., Kawaguchi, T., Berger, M. S. & Pieper, R. O. DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ. 14, 548–558 (2007).
Cosse, J.-P., Rommelaere, G., Ninane, N., Arnould, T. & Michiels, C. BNIP3 protects HepG2 cells against etoposide-induced cell death under hypoxia by an autophagy-independent pathway. Biochem. Pharmacol. 80, 1160–1169 (2010).
Biton, S. & Ashkenazi, A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 145, 92–103 (2011).
Paquet, C., Sané, A.-T., Beauchemin, M. & Bertrand, R. Caspase- and mitochondrial dysfunction-dependent mechanisms of lysosomal leakage and cathepsin B activation in DNA damage-induced apoptosis. Leukemia 19, 784–791 (2005).
Han, J. et al. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J. Biol. Chem. 283, 19665–19677 (2008).
Tristão, V. R. et al. Nec-1 protects against nonapoptotic cell death in cisplatin-induced kidney injury. Ren. Fail. 34, 373–377 (2012).
Liu, D., Yang, Y., Liu, Q. & Wang, J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Medical Oncol. 28, 105–111 (2011).
Bien, S. et al. Doxorubicin-induced cell death requires cathepsin B in HeLa cells. Biochem. Pharmacol. 80, 1466–1477 (2010).
Lin, C.-I. et al. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. MCR 8, 1217–1226 (2010).
Laussmann, M. A. et al. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 18, 1584–1597 (2011).
Yeung, B. H. Y., Huang, D.-C. & Sinicrope, F. A. PS-341 (bortezomib) induces lysosomal cathepsin B release and a caspase-2-dependent mitochondrial permeabilization and apoptosis in human pancreatic cancer cells. J. Biol. Chem. 281, 11923–11932 (2006).
Hu, J. et al. Herceptin conjugates linked by EDC boost direct tumor cell death via programmed tumor cell necrosis. PloS ONE 6, e23270 (2011).
Meurette, O. et al. TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell death under acidic extracellular conditions. Cancer Res. 67, 218–226 (2007).
Nagaraj, N. S., Vigneswaran, N. & Zacharias, W. Cathepsin B mediates TRAIL-induced apoptosis in oral cancer cells. J. Cancer Res. Clin. Oncol. 132, 171–183 (2006).
Herrero-Martín, G. et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 (2009).
Guicciardi, M. E. et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest 106, 1127–1137 (2000).
Djavaheri-Mergny, M. et al. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J. Biol. Chem. 281, 30373–30382 (2006).
Deiss, L. P., Galinka, H., Berissi, H., Cohen, O. & Kimchi, A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 15, 3861–3870 (1996).
Zhang, Y. et al. Fas-mediated autophagy requires JNK activation in HeLa cells. Biochem. Biophys. Res. Comm. 377, 1205–1210 (2008).
Thapa, R. J. et al. NF-κB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol. Cell. Biol. 31, 2934–2946 (2011).
Pyo, J.-O. et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J. Biol. Chem. 280, 20722–20729 (2005).
Gowran, A. & Campbell, V. A. A role for p53 in the regulation of lysosomal permeability by delta 9-tetrahydrocannabinol in rat cortical neurones: implications for neurodegeneration. J. Neurochem. 105, 1513–1524 (2008).
Salazar, M. et al. Cannabinoid action induces autophagy- mediated cell death through stimulation of ER stress in human glioma cells. Autophagy 119, 1359–1372 (2009).
Jang, M.-S., Lee, S.-J., Kang, N. S. & Kim, E. Cooperative phosphorylation of FADD by Aur-A and Plk1 in response to taxol triggers both apoptotic and necrotic cell death. Cancer Res. 71, 7207–7215 (2011).
Zou, C.-F. et al. Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. BMC Cancer 11, 22 (2011).
Xi, G. et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 307, 141–148 (2011).
Löder, S. et al. RIP1 is required for IAP inhibitor-mediated sensitization of childhood acute leukemia cells to chemotherapy-induced apoptosis. Leukemia 16 Dec 2011 (doi: 10.1038/leu.2011.353).
Crazzolara, R. et al. Potentiating effects of RAD001 (Everolimus) on vincristine therapy in childhood acute lymphoblastic leukemia. Blood 113, 3297–3306 (2009).
Stühmer, T. et al. Preclinical anti-myeloma activity of the novel HDAC-inhibitor JNJ-26481585. British J. Haem. 149, 529–536 (2010).
Mitchell, C. et al. Extrinsic pathway- and cathepsin-dependent induction of mitochondrial dysfunction are essential for synergistic flavopiridol and vorinostat lethality in breast cancer cells. Mol. Cancer Therap. 6, 3101–3112 (2007).
Liu, Y.-L. et al. Autophagy potentiates the anti-cancer effects of the histone deacetylase inhibitors in hepatocellular carcinoma. Autophagy 6, 1057–1065 (2010).
Carew, J. S. et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110, 313–322 (2007).
Behrends, C., Sowa, M. E., Gygi, S. P., Harper, J. W. Network organization of the human autophagy system. Nature 466, 68–76 (2010).
Acknowledgements
The authors would like to apologise to their colleagues, whose work they have not been able to cite owing to space limitations. Furthermore, they thank Trinity College Cambridge for financial support of P.K. through a Junior Research Fellowship. Research in the Watson laboratory is funded by the BBSRC, MRC, CRUK, Breast Cancer Campaign and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Kreuzaler, P., Watson, C. Killing a cancer: what are the alternatives?. Nat Rev Cancer 12, 411–424 (2012). https://doi.org/10.1038/nrc3264
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3264
This article is cited by
-
A comprehensive pan-cancer analysis of necroptosis molecules in four gynecologic cancers
BMC Cancer (2022)
-
Identification and validation of necroptosis-related prognostic gene signature and tumor immune microenvironment infiltration characterization in esophageal carcinoma
BMC Gastroenterology (2022)
-
Regulated cell death (RCD) in cancer: key pathways and targeted therapies
Signal Transduction and Targeted Therapy (2022)
-
A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications
Nano-Micro Letters (2021)
-
Cell–cell contacts protect against t-BuOOH-induced cellular damage and ferroptosis in vitro
Archives of Toxicology (2019)