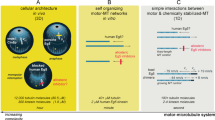
Key Points
-
More than 650 members of the kinesin superfamily have been discovered in eukaryotic organisms. They are categorized into 14 subfamilies on the basis of sequence homology and classified as mitotic kinesins, which are involved in cell division, and non-mitotic kinesins, which are principally involved in intracellular transport.
-
The mitotic spindle is a validated target in cancer chemotherapy, and several agents that target tubulin and that interfere with microtubule dynamics are in clinical use. This success has triggered the search for additional mitotic spindle targets, including mitotic kinases (such as cyclin-dependent kinases, Aurora kinase A, Aurora kinase B and Polo-like kinase 1) and specific mitotic kinesins.
-
EG5 and centromere-associated protein E (CENPE) are two mitotic kinesins that have received much of this attention. Specific inhibitors have been developed and are currently in multiple Phase I or Phase II clinical trials. Other kinesins are also considered potential therapeutic targets and candidate inhibitors have been described.
-
Several non-mitotic kinesins are also involved in tumorigenesis and in the development of resistance to anticancer agents.
-
Several kinesins have multiple functions in mitosis or in intracellular transport. Therefore, careful validation is needed to identify any important side effects and to clarify whether they are viable cancer drug targets.
Abstract
Kinesins are a family of molecular motors that travel unidirectionally along microtubule tracks to fulfil their many roles in intracellular transport or cell division. Over the past few years kinesins that are involved in mitosis have emerged as potential targets for cancer drug development. Several compounds that inhibit two mitotic kinesins (EG5 (also known as KIF11) and centromere-associated protein E (CENPE)) have entered Phase I and II clinical trials either as monotherapies or in combination with other drugs. Additional mitotic kinesins are currently being validated as drug targets, raising the possibility that the range of kinesin-based drug targets may expand in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jordan, M. A. & Wilson, L. Microtubules as a target for anticancer drugs. Nature Rev. Cancer 4, 253–265 (2004).
Orr, G. A., Verdier-Pinard, P., McDaid, H. & Horwitz, S. B. Mechanisms of Taxol resistance related to microtubules. Oncogene 22, 7280–7295 (2003).
Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nature Rev. Cancer 10, 194–204 (2010).
Wood, K. W., Cornwell, W. D. & Jackson, J. R. Past and future of the mitotic spindle as an oncology target. Curr. Opin. Pharmacol. 1, 370–377 (2001).
Miki, H., Okada, Y. & Hirokawa, N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 15, 467–476 (2005).
Hirokawa, N., Niwa, S. & Tanaka, Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68, 610–638 (2010).
Lawrence, C. J. et al. A standardized kinesin nomenclature. J. Cell Biol. 167, 19–22 (2004).
Wordeman, L. How kinesin motor proteins drive mitotic spindle function: lessons from molecular assays. Semin. Cell Dev. Biol. 21, 260–268 (2010).
Hirokawa, N., Noda, Y., Tanaka, Y. & Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nature Rev. Mol. Cell Biol. 10, 682–696 (2009).
Blangy, A. et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169 (1995).
Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999).
Kantarjian, H. M. et al. Phase I/II multicenter study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of AZD4877 in patients with refractory acute myeloid leukemia. Invest. New Drugs 30, 1107–111115 (2011).
Infante, J. R. et al. A Phase I study to assess the safety, tolerability, and pharmacokinetics of AZD4877, an intravenous Eg5 inhibitor in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 69, 165–172 (2011).
Purcell, J. W. et al. Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clin. Cancer Res. 16, 566–576 (2010).
Burris, H. A. et al. A phase I study of ispinesib, a kinesin spindle protein inhibitor, administered weekly for three consecutive weeks of a 28-day cycle in patients with solid tumors. Invest. New Drugs 29, 467–472 (2011).
Knox, J. J. et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepato-cellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168). Invest. New Drugs 26, 265–272 (2008).
Lee, R. T. et al. A University of Chicago consortium phase II trial of SB-715992 in advanced renal cell cancer. Clin. Genitourin Cancer 6, 21–24 (2008).
Holen, K. et al. A phase I trial of MK-0731, a Kinesin Spindle Protein (KSP) inhibitor, in patients with solid tumors. Invest. New Drugs 30, 1088–1095 (2012).
Holen, K. D. et al. A first in human study of SB-743921, a kinesin spindle protein inhibitor, to determine pharmacokinetics, biologic effects and establish a recommended phase II dose. Cancer Chemother. Pharmacol. 67, 447–454 (2011).
Lee, C. W. et al. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest. New Drugs 26, 249–255 (2008).
Beer, T. M. et al. Southwest Oncology Group phase II study of ispinesib in androgen-independent prostate cancer previously treated with taxanes. Clin. Genitourin. Cancer 6, 103–109 (2008).
Tang, P. A. et al. Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Invest. New Drugs 26, 257–264 (2008).
Souid, A. K. et al. A pediatric phase I trial and pharmacokinetic study of ispinesib: a Children's Oncology Group phase I consortium study. Pediatr. Blood Cancer 55, 1323–1328 (2010).
Blagden, S. P. et al. A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br. J. Cancer 98, 894–899 (2008).
Gerecitano, J. F. et al. A Phase I trial of the kinesin spindle protein (Eg5) inhibitor AZD4877 in patients with solid and lymphoid malignancies. Invest. New Drugs 22 May 2012 (doi:10.1007/s10637-012-9821-y).
Miller, K. et al. Phase II, Open label study of ispinesib in patients with locally advanced or metastatic breast cancer. 28th Ann. San Antonio Breast Cancer Symp. Abstr. 1089 (San Antonio, 2005).
Lin, S. et al. Inhibition of Kinesin-5, a microtubule-based motor protein, as a strategy for enhancing regeneration of adult axons. Traffic 12, 269–286 (2011).
Komlodi-Pasztor, E., Sackett, D. L. & Fojo, A. T. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin. Cancer Res. 18, 51–63 (2012).
Sueishi, M., Takagi, M. & Yoneda, Y. The forkhead-associated domain of Ki-67 antigen interacts with the novel kinesin-like protein Hklp2. J. Biol. Chem. 275, 28888–28892 (2000).
Tanenbaum, M. E. et al. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr. Biol. 19, 1703–1711 (2009). This paper identified the functional redundancy of EG5 and KIF15, which provides a possible explanation for the limited success of EG5 inhibitors.
Vanneste, D., Takagi, M., Imamoto, N. & Vernos, I. The role of Hklp2 in the stabilization and maintenance of spindle bipolarity. Curr. Biol. 19, 1712–1717 (2009).
Scanlan, M. J. et al. Humoral immunity to human breast cancer: antigen definition and quantitative analysis of mRNA expression. Cancer Immun. 1, 4 (2001).
Perera, C. N., Spalding, H. S., Mohammed, S. I. & Camarillo, I. G. Identification of proteins secreted from leptin stimulated MCF-7 breast cancer cells: a dual proteomic approach. Exp. Biol. Med. 233, 708–720 (2008).
Nadar, V. C., Ketschek, A., Myers, K. A., Gallo, G. & Baas, P. W. Kinesin-5 is essential for growth-cone turning. Curr. Biol. 18, 1972–1977 (2008).
Liu, M. et al. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J. Neurosci. 30, 14896–14906 (2010).
Jones, S. F. et al. Phase I study of ispinesib in combination with carboplatin in patients with advanced solid tumors. Proc. Am. Soc. Clin. Oncol. Abstr. 24, 2027 (2006).
Rhodon, J. et al. Phase I study of ispinesib (SB-715992), a kinesin spindle protein inhibitor, in combination with capecitabine in patients with advanced solid tumors. Eur. J. Canc. Suppl. 4, 193 (2006).
Yen, T. J. et al. CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 10, 1245–1254 (1991).
Mao, Y., Desai, A. & Cleveland, D. W. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J. Cell Biol. 170, 873–880 (2005).
Kim, Y., Holland, A. J., Lan, W. & Cleveland, D. W. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell 142, 444–455 (2010).
Chan, G. K., Jablonski, S. A., Sudakin, V., Hittle, J. C. & Yen, T. J. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941–954 (1999).
Yang, C. P., Liu, L., Ikui, A. E. & Horwitz, S. B. The interaction between mitotic checkpoint proteins, CENP-E and BubR1, is diminished in epothilone B-resistant A549 cells. Cell Cycle 9, 1207–1213 (2010).
Chanel-Vos, C. & Giannakakou, P. CENP-E checks in microtubule-drug resistance. Cell Cycle 9, 1456–1465 (2010).
Putkey, F. R. et al. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell 3, 351–365 (2002).
Weaver, B. A. & Cleveland, D. W. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 67, 10103–10105 (2007).
Weaver, B. A., Silk, A. D., Montagna, C., Verdier-Pinard, P. & Cleveland, D. W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007). This paper describes the involvement of CENPE in aneuploidy.
Agarwal, R. et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin. Cancer Res. 15, 3654–3662 (2009).
Liu, Z. et al. Reduced expression of cenp-e in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 28, 156 (2009).
Wood, K. W. et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proc. Natl Acad. Sci. USA 107, 5839–5844 (2010). This work describes the mechanism of action of the first CENPE inhibitor that displayed in vivo efficacy.
Wood, K. W., Chua, P., Sutton, D. & Jackson, J. R. Centromere-associated protein E: a motor that puts the brakes on the mitotic checkpoint. Clin. Cancer Res. 14, 7588–7592 (2008).
Chung, V. et al. First-time-in-human study of GSK923295, a novel antimitotic inhibitor of centromere-associated protein E (CENP-E), in patients with refractory cancer. Cancer Chemother. Pharmacol. 69, 733–741 (2012).
Ding, X. et al. Probing CENP-E function in chromosome dynamics using small molecule inhibitor syntelin. Cell Res. 20, 1386–1389 (2010).
Huang, Y. et al. Defects in chromosome congression and mitotic progression in KIF18A-deficient cells are partly mediated through impaired functions of CENP-E. Cell Cycle 8, 2643–2649 (2009).
Sharp, D. J., Rogers, G. C. & Scholey, J. M. Microtubule motors in mitosis. Nature 407, 41–47 (2000).
Ando, A. et al. Cloning of a new kinesin-related gene located at the centromeric end of the human MHC region. Immunogenetics 39, 194–200 (1994).
DeLuca, J. G., Newton, C. N., Himes, R. H., Jordan, M. A. & Wilson, L. Purification and characterization of native conventional kinesin, HSET, and CENP-E from mitotic hela cells. J. Biol. Chem. 276, 28014–28021 (2001).
Mountain, V. et al. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 147, 351–366 (1999).
Zhu, C. et al. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Mol. Biol. Cell 16, 3187–3199 (2005). This work describes a detailed study identifying 12 members of the kinesin superfamily involved in mitosis and cytokinesis using RNAi and high-resolution imaging.
Cai, S., Weaver, L. N., Ems-McClung, S. C. & Walczak, C. E. Kinesin-14 family proteins HSET/XCTK2 control spindle length by cross-linking and sliding microtubules. Mol. Biol. Cell 20, 1348–1359 (2009).
Cai, S., Weaver, L. N., Ems-McClung, S. C. & Walczak, C. E. Proper organization of microtubule minus ends is needed for midzone stability and cytokinesis. Curr. Biol. 20, 880–885 (2010).
Grinberg-Rashi, H. et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin. Cancer Res. 15, 1755–1761 (2009).
De, S., Cipriano, R., Jackson, M. W. & Stark, G. R. Overexpression of kinesins mediates docetaxel resistance in breast cancer cells. Cancer Res. 69, 8035–8042 (2009).
Kwon, M. et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 22, 2189–2203 (2008). This work identified the role of KIFC1 in the chromosome clustering phenomenon that allows cancer cells to divide and evade cell death despite the presence of extra centrosomes.
Chandhok, N. S. & Pellman, D. A little CIN may cost a lot: revisiting aneuploidy and cancer. Curr. Opin. Genet. Dev. 19, 74–81 (2009).
Ems-McClung, S. C. & Walczak, C. E. Kinesin-13s in mitosis: key players in the spatial and temporal organization of spindle microtubules. Semin. Cell Dev. Biol. 21, 276–282 (2010).
Jang, C. Y. et al. Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J. Cell Sci. 122, 1334–1341 (2009).
Knowlton, A. L., Vorozhko, V. V., Lan, W., Gorbsky, G. J. & Stukenberg, P. T. ICIS and Aurora B coregulate the microtubule depolymerase Kif2a. Curr. Biol. 19, 758–763 (2009).
Hood, E. A., Kettenbach, A. N., Gerber, S. A. & Compton, D. A. Plk1 regulates the kinesin-13 protein Kif2b to promote faithful chromosome segregation. Mol. Biol. Cell 25 Apr 2012 (doi:10.1091/mbc.E11-12-1013).
Tanenbaum, M. E., Medema, R. H. & Akhmanova, A. Regulation of localization and activity of the microtubule depolymerase MCAK. Bioarchitecture 1, 80–87 (2011).
Ganem, N. J. & Compton, D. A. The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J. Cell Biol. 166, 473–478 (2004).
Homma, N. et al. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114, 229–239 (2003).
Wang, C. Q. et al. Overexpression of Kif2a promotes the progression and metastasis of squamous cell carcinoma of the oral tongue. Oral Oncol. 46, 65–69 (2010).
Schimizzi, G. V., Currie, J. D. & Rogers, S. L. Expression levels of a kinesin-13 microtubule depolymerase modulates the effectiveness of anti-microtubule agents. PLoS ONE 5, e11381 (2010).
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biol. 11, 27–35 (2009).
Cimini, D., Moree, B., Canman, J. C. & Salmon, E. D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213–4225 (2003).
Manning, A. L. et al. The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol. Biol. Cell 18, 2970–2979 (2007).
Moore, A. T. et al. MCAK associates with the tips of polymerizing microtubules. J. Cell Biol. 169, 391–397 (2005).
Bakhoum, S. F., Genovese, G. & Compton, D. A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937–1942 (2009).
Goldie, J. H. & Coldman, A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 63, 1727–1733 (1979).
Ishikawa, K. et al. Mitotic centromere-associated kinesin is a novel marker for prognosis and lymph node metastasis in colorectal cancer. Br. J. Cancer 98, 1824–1829 (2008).
Nakamura, Y. et al. Clinicopathological and biological significance of mitotic centromere-associated kinesin overexpression in human gastric cancer. Br. J. Cancer 97, 543–549 (2007).
Shimo, A. et al. Involvement of kinesin family member 2C/mitotic centromere-associated kinesin overexpression in mammary carcinogenesis. Cancer Sci. 99, 62–70 (2008).
Shimo, A. et al. Elevated expression of protein regulator of cytokinesis 1, involved in the growth of breast cancer cells. Cancer Sci. 98, 174–181 (2007).
Ganguly, A., Yang, H. & Cabral, F. Overexpression of mitotic centromere-associated Kinesin stimulates microtubule detachment and confers resistance to paclitaxel. Mol. Cancer Ther. 10, 929–937 (2011).
Ganguly, A., Yang, H., Pedroza, M., Bhattacharya, R. & Cabral, F. Mitotic centromere-associated kinesin (MCAK) mediates paclitaxel resistance. J. Biol. Chem. 286, 36378–36384 (2011).
Gonzalez-Garay, M. L., Chang, L., Blade, K., Menick, D. R. & Cabral, F. A β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J. Biol. Chem. 274, 23875–23882 (1999).
Hedrick, D. G., Stout, J. R. & Walczak, C. E. Effects of anti-microtubule agents on microtubule organization in cells lacking the kinesin-13 MCAK. Cell Cycle 7, 2146–2156 (2008).
Aoki, S., Ohta, K., Yamazaki, T., Sugawara, F. & Sakaguchi, K. Mammalian mitotic centromere-associated kinesin (MCAK): a new molecular target of sulfoquinovosylacylglycerols novel antitumor and immunosuppressive agents. FEBS J. 272, 2132–2140 (2005).
Rickert, K. W. et al. Discovery and biochemical characterization of selective ATP competitive inhibitors of the human mitotic kinesin KSP. Arch. Biochem. Biophys. 469, 220–231 (2008).
Mazumdar, M. & Misteli, T. Chromokinesins: multitalented players in mitosis. Trends Cell Biol. 15, 349–355 (2005).
Zhu, C. & Jiang, W. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc. Natl Acad. Sci. USA 102, 343–348 (2005).
Kurasawa, Y., Earnshaw, W. C., Mochizuki, Y., Dohmae, N. & Todokoro, K. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 23, 3237–3248 (2004).
Mazumdar, M., Sundareshan, S. & Misteli, T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 166, 613–620 (2004).
Mazumdar, M. et al. Tumor formation via loss of a molecular motor protein. Curr. Biol. 16, 1559–1564 (2006). The authors show that loss of KIF4 leads to aneuploidy and subsequent induction of tumorigenesis in vitro and in vivo.
Narayan, G. et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosom. Cancer 46, 373–384 (2007).
Taniwaki, M. et al. Activation of KIF4A as a prognostic biomarker and therapeutic target for lung cancer. Clin. Cancer Res. 13, 6624–6631 (2007).
Rouam, S., Moreau, T. & Broet, P. Identifying common prognostic factors in genomic cancer studies: a novel index for censored outcomes. BMC Bioinformatics 11, 150 (2010).
Gao, J. et al. Overexpression of chromokinesin KIF4 inhibits proliferation of human gastric carcinoma cells both in vitro and in vivo. Tumour Biol. 32, 53–61 (2011).
Lee, Y. M. & Kim, W. Association of human kinesin superfamily protein member 4 with BRCA2-associated factor 35. Biochem. J. 374, 497–503 (2003).
Wu, G. et al. A novel role of the chromokinesin Kif4A in DNA damage response. Cell Cycle 7, 2013–2020 (2008).
Midorikawa, R., Takei, Y. & Hirokawa, N. KIF4 motor regulates activity-dependent neuronal survival by suppressing PARP-1 enzymatic activity. Cell 125, 371–383 (2006).
Mayr, M. I. et al. The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr. Biol. 17, 488–498 (2007).
Tokai, N. et al. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J. 15, 457–467 (1996).
Stumpff, J., von Dassow, G., Wagenbach, M., Asbury, C. & Wordeman, L. The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev. Cell 14, 252–262 (2008).
Stumpff, J. et al. A tethering mechanism controls the processivity and kinetochore-microtubule plus-end enrichment of the kinesin-8 Kif18A. Mol. Cell 43, 764–775 (2011).
Zhang, C. et al. Kif18A is involved in human breast carcinogenesis. Carcinogenesis 31, 1676–1684 (2010).
Nagahara, M. et al. Kinesin 18A expression: clinical relevance to colorectal cancer progression. Int. J. Cancer 129, 2543–2552 (2011).
Catarinella, M., Gruner, T., Strittmatter, T., Marx, A. & Mayer, T. U. BTB-1: a small molecule inhibitor of the mitotic motor protein Kif18A. Angew. Chem. Int. Ed Engl. 48, 9072–9076 (2009).
Lee, Y. M. et al. Cell cycle-regulated expression and subcellular localization of a kinesin-8 member human KIF18B. Gene 466, 16–25 (2010).
Stout, J. R. et al. Kif18B interacts with EB1 and controls astral microtubule length during mitosis. Mol. Biol. Cell 22, 3070–3080 (2011).
Tanenbaum, M. E. et al. A complex of Kif18b and MCAK promotes microtubule depolymerization and is negatively regulated by Aurora kinases. Curr. Biol. 21, 1356–1365 (2011).
Shiroguchi, K., Ohsugi, M., Edamatsu, M., Yamamoto, T. & Toyoshima, Y. Y. The second microtubule-binding site of monomeric Kid enhances the microtubule affinity. J. Biol. Chem. 278, 22460–22465 (2003).
Levesque, A. A. & Compton, D. A. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 154, 1135–1146 (2001).
Ohsugi, M. et al. Cdc2-mediated phosphorylation of Kid controls its distribution to spindle and chromosomes. EMBO J. 22, 2091–2103 (2003).
Brouhard, G. J. & Hunt, A. J. Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro. Proc. Natl Acad. Sci. USA 102, 13903–13908 (2005).
Santamaria, A., Nagel, S., Sillje, H. H. & Nigg, E. A. The spindle protein CHICA mediates localization of the chromokinesin Kid to the mitotic spindle. Curr. Biol. 18, 723–729 (2008).
Ohsugi, M. et al. Kid-mediated chromosome compaction ensures proper nuclear envelope formation. Cell 132, 771–782 (2008).
Tokai-Nishizumi, N., Ohsugi, M., Suzuki, E. & Yamamoto, T. The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol. Biol. Cell 16, 5455–5463 (2005).
Eggert, U. S. et al. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2, e379 (2004).
Eggert, U. S., Mitchison, T. J. & Field, C. M. Animal cytokinesis: from parts list to mechanisms. Annu. Rev. Biochem. 75, 543–566 (2006).
Carleton, M. et al. RNA interference-mediated silencing of mitotic kinesin KIF14 disrupts cell cycle progression and induces cytokinesis failure. Mol. Cell. Biol. 26, 3853–3863 (2006).
Gruneberg, U. et al. KIF14 and citron kinase act together to promote efficient cytokinesis. J. Cell Biol. 172, 363–372 (2006).
Corson, T. W. & Gallie, B. L. KIF14 mRNA expression is a predictor of grade and outcome in breast cancer. Int. J. Cancer 119, 1088–1094 (2006).
Corson, T. W. et al. KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clin. Cancer Res. 13, 3229–3234 (2007).
Theriault, B. L., Pajovic, S., Bernardini, M. Q., Shaw, P. A. & Gallie, B. L. Kinesin family member 14: an independent prognostic marker and potential therapeutic target for ovarian cancer. Int. J. Cancer 130, 1844–1854 (2011).
Corson, T. W., Huang, A., Tsao, M. S. & Gallie, B. L. KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene 24, 4741–4753 (2005).
Nislow, C., Lombillo, V. A., Kuriyama, R. & McIntosh, J. R. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature 359, 543–547 (1992).
Mishima, M., Kaitna, S. & Glotzer, M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41–54 (2002).
Zhu, C., Bossy-Wetzel, E. & Jiang, W. Recruitment of MKLP1 to the spindle midzone/midbody by INCENP is essential for midbody formation and completion of cytokinesis in human cells. Biochem. J. 389, 373–381 (2005).
Lee, K. S., Yuan, Y. L., Kuriyama, R. & Erikson, R. L. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol. Cell. Biol. 15, 7143–7151 (1995).
Liu, X., Zhou, T., Kuriyama, R. & Erikson, R. L. Molecular interactions of Polo-like-kinase 1 with the mitotic kinesin-like protein CHO1/MKLP-1. J. Cell Sci. 117, 3233–3246 (2004).
Neef, R., Klein, U. R., Kopajtich, R. & Barr, F. A. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr. Biol. 16, 301–307 (2006).
Takahashi, S. et al. Downregulation of KIF23 suppresses glioma proliferation. J. Neurooncol. 106, 519–529 (2012).
Valk, K. et al. Gene expression profiles of non-small cell lung cancer: survival prediction and new biomarkers. Oncology 79, 283–292 (2010).
Wang, S. M., Ooi, L. L. & Hui, K. M. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin. Cancer Res. 17, 6040–6051 (2011).
Echard, A. et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 279, 580–585 (1998).
Fontijn, R. D. et al. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol. Cell. Biol. 21, 2944–2955 (2001).
Hill, E., Clarke, M. & Barr, F. A. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19, 5711–5719 (2000).
Simizu, S. & Osada, H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nature Cell Biol. 2, 852–854 (2000).
Neef, R. et al. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863–875 (2003).
Gruneberg, U., Neef, R., Honda, R., Nigg, E. A. & Barr, F. A. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166, 167–172 (2004).
Huemmer, S. & Mayer, T. Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr. Biol. 19, 607–612 (2009).
Taniuchi, K. et al. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 65, 105–112 (2005).
Kamimoto, T., Zama, T., Aoki, R., Muro, Y. & Hagiwara, M. Identification of a novel kinesin-related protein, KRMP1, as a target for mitotic peptidyl-prolyl isomerase Pin1. J. Biol. Chem. 276, 37520–37528 (2001).
Kanehira, M. et al. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Res. 67, 3276–3285 (2007).
Abaza, A. et al. M phase phosphoprotein 1 is a human plus-end-directed kinesin-related protein required for cytokinesis. J. Biol. Chem. 278, 27844–27852 (2003).
Krzywicka-Racka, A. & Sluder, G. Repeated cleavage failure does not establish centrosome amplification in untransformed human cells. J. Cell Biol. 194, 199–207 (2011).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005).
Ng, J. M. & Curran, T. The Hedgehog's tale: developing strategies for targeting cancer. Nature Rev. Cancer 11, 493–501 (2011).
Wilson, C. W. & Chuang, P. T. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137, 2079–2094 (2010).
Endoh-Yamagami, S. et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr. Biol. 19, 1320–1326 (2009).
Wong, S. Y. et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nature Med. 15, 1055–1061 (2009).
Cheung, H. O. et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci. Signal. 2, ra29 (2009).
Hui, C. C. & Angers, S. Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol. 27, 513–537 (2011).
Varjosalo, M. & Taipale, J. Hedgehog: functions and mechanisms. Genes Dev. 22, 2454–2472 (2008).
Hirokawa, N. Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1, 29–34 (2000).
Huangfu, D. & Anderson, K. V. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3–14 (2006).
Kovacs, J. J. et al. β-arrestin-mediated localization of smoothened to the primary cilium. Science 320, 1777–1781 (2008).
Kolpakova-Hart, E., Jinnin, M., Hou, B., Fukai, N. & Olsen, B. R. Kinesin-2 controls development and patterning of the vertebrate skeleton by Hedgehog- and Gli3-dependent mechanisms. Dev. Biol. 309, 273–284 (2007).
Score, J. et al. Identification of a novel imatinib responsive KIF5B-PDGFRA fusion gene following screening for PDGFRA overexpression in patients with hypereosinophilia. Leukemia 20, 827–832 (2006).
Takeuchi, K. et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin. Cancer Res. 15, 3143–3149 (2009).
Wong, D. W. et al. A novel KIF5B-ALK variant in nonsmall cell lung cancer. Cancer 117, 2709–2718 (2011).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Tan, M. H. et al. Specific kinesin expression profiles associated with taxane resistance in basal-like breast cancer. Breast Cancer Res. Treat. 131, 849–858 (2011).
Okada, Y., Yamazaki, H., Sekine-Aizawa, Y. & Hirokawa, N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780 (1995).
Loyo, M. et al. A survey of methylated candidate tumor suppressor genes in nasopharyngeal carcinoma. Int. J. Cancer 128, 1393–1403 (2011).
Ostrow, K. L. et al. Molecular analysis of plasma DNA for the early detection of lung cancer by quantitative methylation-specific PCR. Clin. Cancer Res. 16, 3463–3472 (2010).
Cheung, I. Y., Feng, Y., Gerald, W. & Cheung, N. K. Exploiting gene expression profiling to identify novel minimal residual disease markers of neuroblastoma. Clin. Cancer Res. 14, 7020–7027 (2008).
Yeh, I. T. et al. A germline mutation of the KIF1B β gene on 1p36 in a family with neural and nonneural tumors. Hum. Genet. 124, 279–285 (2008).
Zhang, H. et al. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nature Genet. 42, 755–758 (2010).
Matsuno, K., Sawada, J. S. & Asai, A. Therapeutic potential of mitotic kinesin inhibitors in cancer. Expert Opin. Ther. Patents 18, 253–274 (2008).
McDonald, A., Bergnes, G. & Morgans, D. J. US Patents 514/266.2; 514/266.23; 514/309; 544/284 and 546/142 (2004).
Good, J. A., Skoufias, D. A. & Kozielski, F. Elucidating the functionality of kinesins: an overview of small molecule inhibitors. Semin. Cell Dev. Biol. 22, 935–945 (2011).
Tcherniuk, S. et al. Relocation of Aurora B and survivin from centromeres to the central spindle impaired by a kinesin-specific MKLP-2 inhibitor. Angew. Chem. Int. Ed Engl. 49, 8228–8231 (2010).
Roche, H. et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J. Clin. Oncol. 25, 3415–3420 (2007).
Perez, E. A. et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 25, 3407–3414 (2007).
Cavaletti, G. & Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Nature Rev. Neurol. 6, 657–666 (2010).
Ibrahim, N. K. et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J. Clin. Oncol. 23, 6019–6026 (2005).
Gradishar, W. J. et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 23, 7794–7803 (2005).
Jackson, J. R., Patrick, D. R., Dar, M. M. & Huang, P. S. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nature Rev. Cancer 7, 107–117 (2007).
Strebhardt, K. & Ullrich, A. Targeting polo-like kinase 1 for cancer therapy. Nature Rev. Cancer 6, 321–330 (2006).
Lens, S. M., Voest, E. E. & Medema, R. H. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nature Rev. Cancer 10, 825–841 (2010).
Dar, A. A., Goff, L. W., Majid, S., Berlin, J. & El-Rifai, W. Aurora kinase inhibitors--rising stars in cancer therapeutics? Mol. Cancer Ther. 9, 268–278 (2010).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Rev. Cancer 9, 153–166 (2009).
Barsanti, P. A. et al. The discovery of tetrahydro-β-carbolines as inhibitors of the kinesin Eg5. Bioorg. Med. Chem. Lett. 20, 157–160 (2010).
Cox, C. D. et al. Kinesin spindle protein (KSP) inhibitors. 9. Discovery of (2S)-4-(2,5-difluorophenyl)-n-[(3R,4S)-3-fluoro-1-methylpiperidin-4-yl]-2- (hydroxymethyl)-N-methyl-2-phenyl-2,5-dihydro-1H-pyrrole-1-carboxamide (MK-0731) for the treatment of taxane-refractory cancer. J. Med. Chem. 51, 4239–4252 (2008).
Gartner, M. et al. Development and biological evaluation of potent and specific inhibitors of mitotic Kinesin Eg5. Chembiochem 6, 1173–1177 (2005).
Kim, K. S. et al. Synthesis and SAR of pyrrolotriazine-4-one based Eg5 inhibitors. Bioorg. Med. Chem. Lett. 16, 3937–3942 (2006).
Nakai, R. et al. K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res. 69, 3901–3909 (2009).
Pinkerton, A. B. et al. Synthesis and SAR of thiophene containing kinesin spindle protein (KSP) inhibitors. Bioorg. Med. Chem. Lett. 17, 3562–3569 (2007).
Schiemann, K. et al. The discovery and optimization of hexahydro-2H-pyrano[3,2-c]quinolines (HHPQs) as potent and selective inhibitors of the mitotic kinesin-5. Bioorg. Med. Chem. Lett. 20, 1491–1495 (2010).
Wang, F. et al. Triphenylbutanamines: kinesin spindle protein inhibitors with in vivo antitumor activity. J. Med. Chem. 55, 1511–1525 (2012).
Sakowicz, R. et al. Antitumor activity of a kinesin inhibitor. Cancer Res. 64, 3276–3280 (2004). This article was the first to describe in vivo activity of an EG5 inhibitor, thus manifesting kinesins as drug targets for cancer therapy.
Yan, Y. et al. Inhibition of a mitotic motor protein: where, how, and conformational consequences. J. Mol. Biol. 335, 547–554 (2004). This work presents the first crystal structure of an EG5 inhibitor complex and revealed details into the mechanism of action of this class of compounds.
Luo, L. et al. ATP-competitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nature Chem. Biol. 3, 722–726 (2007).
Parrish, C. A. et al. Novel ATP-competitive kinesin spindle protein inhibitors. J. Med. Chem. 50, 4939–4952 (2007).
Learman, S. S. et al. NSC 622124 inhibits human Eg5 and other kinesins via interaction with the conserved microtubule-binding site. Biochemistry 48, 1754–1762 (2009).
Kaan, H. Y., Ulaganathan, V., Hackney, D. D. & Kozielski, F. An allosteric transition trapped in an intermediate state of a new kinesin-inhibitor complex. Biochem. J. 425, 55–60 (2009).
Maliga, Z. et al. A pathway of structural changes produced by monastrol binding to Eg5. J. Biol. Chem. 281, 7977–7982 (2006).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008). The authors present an intriguing systematic study that demonstrated the variability of cell fate after treatment with antimitotic drugs.
Shi, J., Orth, J. D. & Mitchison, T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 68, 3269–3276 (2008).
Garcia-Saez, I., Yen, T., Wade, R. H. & Kozielski, F. Crystal structure of the motor domain of the human kinetochore protein CENP-E. J. Mol. Biol. 340, 1107–1116 (2004).
Turner, J. et al. Crystal structure of the mitotic spindle kinesin Eg5 reveals a novel conformation of the neck-linker. J. Biol. Chem. 276, 25496–25502 (2001).
Parke, C. L., Wojcik, E. J., Kim, S. & Worthylake, D. K. ATP hydrolysis in Eg5 kinesin involves a catalytic two-water mechanism. J. Biol. Chem. 285, 5859–5867 (2010).
Peters, C. et al. Insight into the molecular mechanism of the multitasking kinesin-8 motor. EMBO J. 29, 3437–3447 (2010).
Matuliene, J. & Kuriyama, R. Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol. Biol. Cell 13, 1832–1845 (2002).
Nigg, E. A., Mayer, T. U., Huemmer, S., Barr, F. & Bormann, J. EP1683523 (2006).
Hotha, S. et al. HR22C16: a potent small-molecule probe for the dynamics of cell division. Angew. Chem. Int. Ed Engl. 42, 2379–2382 (2003).
Nowicki, M. O. et al. Chronic myelogenous leukemia molecular signature. Oncogene 22, 3952–3963 (2003).
Liu, M. et al. Ectopic expression of the microtubule-dependent motor protein Eg5 promotes pancreatic tumourigenesis. J. Pathol. 221, 221–228 (2010).
Imai, K. et al. Identification of HLA-A2-restricted CTL epitopes of a novel tumour-associated antigen, KIF20A, overexpressed in pancreatic cancer. Br. J. Cancer 104, 300–307 (2011).
Acknowledgements
The authors would like to thank Cancer Research UK for financial support. They regret that they were unable to include the work of many groups who have contributed to the understanding of the functions of various kinesins from non-human species.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Consultancy (F.K.); Honoraria for speaking (F.K.); Grants or support for research (F.K.); and Patents (F.K.).
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1
Human kinesin nomenclature and function (PDF 451 kb)
Supplementary information S2
Published work describing kinesin-targeting inhibitors in clinical development (PDF 353 kb)
Supplementary information S3
Human mitotic kinesins and their motor classification. (PDF 393 kb)
Glossary
- Monoastral spindle phenotype
-
An array of microtubules surrounded by a ring of chromosomes.
- Merotelic kinetochore attachment
-
A single kinetochore becomes attached to microtubules from both spindle poles.
- Optical tweezers
-
An instrument that uses a highly focused laser beam to manipulate microscopic objects (for example, biopolymers such as microtubules or molecular motors) and to measure forces.
- Endoreduplication
-
Replication of the genome in the absence of cell division resulting in polyploidy.
- Hypereosinophilia
-
A haematopoietic disease characterized by an unusually high eosinophil count.
Rights and permissions
About this article
Cite this article
Rath, O., Kozielski, F. Kinesins and cancer. Nat Rev Cancer 12, 527–539 (2012). https://doi.org/10.1038/nrc3310
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3310
This article is cited by
-
The role of kinesin family members in hepatobiliary carcinomas: from bench to bedside
Biomarker Research (2024)
-
Regulation of ciliary homeostasis by intraflagellar transport-independent kinesins
Cell Death & Disease (2024)
-
KIF18A as a potential biomarker to distinguish different breast cancer subtypes based on receptor status
Genome Instability & Disease (2024)
-
A co-formulation of interferons alpha2b and gamma distinctively targets cell cycle in the glioblastoma-derived cell line U-87MG
BMC Cancer (2023)
-
Network and functional analyses of differentially expressed genes in gastric cancer provide new biomarkers associated with disease pathogenesis
Journal of the Egyptian National Cancer Institute (2023)