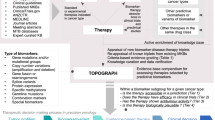
Abstract
If we were to summarize the rationale that underpins medical oncology in a Latin aphorism, it might be 'veneno ergo sum'; that is, I poison, therefore I am. The burden of chemotherapy-associated toxicity is well recognized, but we have relatively few tools that increase the precision of anticancer drug prescribing. We propose a shift in emphasis from the focussed study of polymorphisms in drug metabolic pathways in small sets of patients to broader agnostic analyses to systematically correlate germline genetic variants with adverse events in large, well-defined cancer populations. Thus, we propose the new science of 'toxgnostics' (that is, the systematic, agnostic study of genetic predictors of toxicity from anticancer therapy).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gray, R. et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370, 2020–2029 (2007).
Widakowich, C. et al. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist 12, 1443–1455 (2007).
Bertagnolli, M. M. et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer—a study of CALGB 9581 and 89803. J. Clin. Oncol. 29, 3153–3162 (2011).
Roth, A. D. et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J. Clin. Oncol. 28, 466–474 (2010).
Hutchins, G. et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 29, 1261–1270 (2011).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366, 707–714 (2012).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Parkinson, D. R., Johnson, B. E. & Sledge, G. W. Making personalized cancer medicine a reality: challenges and opportunities in the development of biomarkers and companion diagnostics. Clin. Cancer Res. 18, 619–624 (2012).
Walther, A. et al. Genetic prognostic and predictive markers in colorectal cancer. Nature Rev. Cancer 9, 489–499 (2009).
Church, D., Midgley, R. & Kerr, D. Biomarkers in early-stage colorectal cancer: ready for prime time? Digestive Diseases 30 (Suppl. 2), 27–33 (2012).
Buyse, M., Sargent, D. J., Grothey, A., Matheson, A. & de Gramont, A. Biomarkers and surrogate end points—the challenge of statistical validation. Nature Rev. Clin. Oncol. 7, 309–317 (2010).
Simon, R. M., Paik, S. & Hayes, D. F. Use of Archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl Cancer Institute 101, 1446–1452 (2009).
Ciccolini, J., Gross, E., Dahan, L., Lacarelle, B. & Mercier, C. Routine dihydropyrimidine dehydrogenase testing for anticipating 5-fluorouracil-related severe toxicities: hype or hope? Clin. Colorectal Cancer 9, 224–228 (2010).
Locker, G. Y. et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 24, 5313–5327 (2006).
Gold, H. T., Hall, M. J., Blinder, V. & Schackman, B. R. Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer 115, 3858–3867 (2009).
Schmoll, H. J. et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. 23, 2479–2516 (2012).
Delea, T. E. et al. The incidence and cost of hospitalization for 5-FU toxicity among Medicare beneficiaries with metastatic colorectal cancer. Value Health 5, 35–43 (2002).
Calhoun, E. A. et al. Evaluating the total costs of chemotherapy-induced toxicity: results from a pilot study with ovarian cancer patients. Oncologist 6, 441–445 (2001).
Wishart, D. S. Improving early drug discovery through ADME modelling: an overview. Drugs R. D. 8, 349–362 (2007).
Gamelin, E. et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J. Clin. Oncol. 16, 1470–1478 (1998).
Gamelin, E. C. et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 77, 441–451 (1996).
Hillcoat, B. L., McCulloch, P. B., Figueredo, A. T., Ehsan, M. H. & Rosenfeld, J. M. Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br. J. Cancer 38, 719–724 (1978).
Di Paolo, A. et al. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann. Oncol. 12, 1301–1306 (2001).
Newell, D. R. Clinical pharmacokinetics of antitumor antifolates. Semin. Oncol. 26, 74–81 (1999).
Jolivet, J., Cowan, K. H., Curt, G. A., Clendeninn, N. J. & Chabner, B. A. The pharmacology and clinical use of methotrexate. N. Engl. J. Med. 309, 1094–1104 (1983).
Calvert, A. H. et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 7, 1748–1756 (1989).
Lehmann, H. & Ryan, E. The familial incidence of low pseudocholinesterase level. Lancet 271, 124 (1956).
Alving, A. S., Carson, P. E., Flanagan, C. L. & Ickes, C. E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 124, 484–485 (1956).
Vogel, F. Moderne Probleme der Humangenetik. Ergebn. Inn. Med. Kinderheilkd. 12, 52–125 (1959).
Lennard, L., Lilleyman, J. S., Van Loon, J. & Weinshilboum, R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet 336, 225–229 (1990).
Relling, M. V. et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 89, 387–391 (2011).
Ford, L. T. & Berg, J. D. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J. Clin. Pathol. 63, 288–295 (2010).
Ross, C. J. et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nature Genet. 41, 1345–1349 (2009).
Pussegoda, K. et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin. Pharmacol. Ther. 94, 243–251 (2013).
Yang, J. J. et al. The role of inherited TPMT and COMT genetic variation in cisplatin-induced ototoxicity in children with cancer. Clin. Pharmacol. Ther. 94, 252–259 (2013).
Ratain, M. J., Cox, N. J. & Henderson, T. O. Challenges in interpreting the evidence for genetic predictors of ototoxicity. Clin. Pharmacol. Ther. 94, 631–635 (2013).
Zanger, U. M. & Schwab, M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 138, 103–141 (2013).
Mahgoub, A., Idle, J. R., Dring, L. G., Lancaster, R. & Smith, R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet 2, 584–586 (1977).
Heim, M. & Meyer, U. A. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet 336, 529–532 (1990).
Kupfer, A. & Preisig, R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur. J. Clin. Pharmacol. 26, 753–759 (1984).
Sullivan-Klose, T. H. et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6, 341–349 (1996).
Jonas, D. E. & McLeod, H. L. Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol. Sci. 30, 375–386 (2009).
Schwab, M. & Schaeffeler, E. Warfarin pharmacogenetics meets clinical use. Blood 118, 2938–2939 (2011).
Klein, T. E. et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 360, 753–764 (2009).
Schroth, W. et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J. Clin. Oncol. 25, 5187–5193 (2007).
Hertz, D. L. et al. CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res. Treat. 134, 401–410 (2012).
Hertz, D. L. et al. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann. Oncol. 24, 1472–1478 (2013).
Iyer, L. et al. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J. Clin. Invest. 101, 847–854 (1998).
Ando, Y. et al. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann. Oncol. 9, 845–847 (1998).
Hoskins, J. M., Goldberg, R. M., Qu, P., Ibrahim, J. G. & McLeod, H. L. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J. Natl Cancer Inst. 99, 1290–1295 (2007).
Schwab, M. et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J. Clin. Oncol. 26, 2131–2138 (2008).
Loganayagam, A. et al. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br. J. Cancer 108, 2505–2515 (2013).
McLeod, H. L. et al. Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741. J. Clin. Oncol. 28, 3227–3233 (2010).
Burmeister, H., Aebi, S., Studer, C., Fey, M. F. & Gautschi, O. Adherence to ESMO clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting. Supportive Care Cancer 20, 141–147 (2012).
Watanabe, R. M. Statistical issues in gene association studies. Methods Mol. Biol. 700, 17–36 (2011).
Dunlop, M. G. et al. Common variation near CDKN1A, POLD3 and SHROOM2 influences colorectal cancer risk. Nature Genet. 44, 770–776 (2012).
Houlston, R. S. et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nature Genet. 42, 973–977 (2010).
Daly, A. K. et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nature Genet. 41, 816–819 (2009).
McCormack, M. et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 364, 1134–1143 (2011).
Link, E. et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 359, 789–799 (2008).
Trevino, L. R. et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J. Clin. Oncol. 27, 5972–5978 (2009).
Ramsey, L. B. et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 22, 1–8 (2012).
Kiyotani, K. et al. A genome-wide association study identifies four genetic markers for hematological toxicities in cancer patients receiving gemcitabine therapy. Pharmacogenet. Genomics 22, 229–235 (2012).
Srinivasan, Y. et al. Genome-wide association study of epirubicin-induced leukopenia in Japanese patients. Pharmacogenet. Genomics 21, 552–558 (2011).
Meyerson, M. et al. Advances in understanding cancer genomes through second-generation sequencing. Nat. Rev. Genet. 11, 685–696 (2010).
McDermott, U., Downing, J. R. & Stratton, M. R. Genomics and the continuum of cancer care. N. Engl. J. Med. 364, 340–350 (2011).
Liu, J. et al. Predictive Power Estimation Algorithm (PPEA)—a new algorithm to reduce overfitting for genomic biomarker discovery. PLoS ONE 6, e24233 (2011).
McShane, L. M. et al. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK). J. Natl Cancer Institute 97, 1180–1184 (2005).
Riley, R. D. et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 10, e1001380 (2013).
Martinez-Balibrea, E. et al. UGT1A and TYMS genetic variants predict toxicity and response of colorectal cancer patients treated with first-line irinotecan and fluorouracil combination therapy. Br. J. Cancer 103, 581–589 (2010).
Glimelius, B. et al. Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenomics J. 11, 61–71 (2011).
Cecchin, E. et al. Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J. Clin. Oncol. 27, 2457–2465 (2009).
Pharoah, P. D., Abraham, J. & Caldas, C. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1–98 trial and Re: CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J. Natl. Cancer Inst. 104, 1263–1264; author reply 1266–1268 (2012).
Regan, M. M. et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trial. J. Natl Cancer Inst. 104, 441–451 (2012).
Salazar, R. et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J. Clin. Oncol. 29, 17–24 (2011).
Gray, R. G. et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J. Clin. Oncol. 29, 4611–4619 (2011).
Rosmarin, D. et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-Analysis. J. Clin. Oncol. 32, 1031–1039 (2014).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nature Rev. Cancer 3, 330–338 (2003).
Diasio, R. B., Beavers, T. L. & Carpenter, J. T. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J. Clin. Invest. 81, 47–51 (1988).
Harris, B. E., Carpenter, J. T. & Diasio, R. B. Severe 5-fluorouracil toxicity secondary to dihydropyrimidine dehydrogenase deficiency. A potentially more common pharmacogenetic syndrome. Cancer 68, 499–501 (1991).
Etienne, M. C. et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J. Clin. Oncol. 12, 2248–2253 (1994).
van Staveren, M. C., Jan Guchelaar, H., van Kuilenburg, A. B., Gelderblom, H. & Maring, J. G. Evaluation of predictive tests for screening for dihydropyrimidine dehydrogenase deficiency. Pharmacogenomics J. 13, 389–395 (2013).
Pullarkat, S. T. et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 1, 65–70 (2001).
Fernandez-Rozadilla, C. et al. Pharmacogenomics in colorectal cancer: a genome-wide association study to predict toxicity after 5-fluorouracil or FOLFOX administration. Pharmacogenomics J. 13, 209–217 (2013).
Baldwin, R. M. et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin. Cancer Res. 18, 5099–5109 (2012).
Twelves, C. et al. Capecitabine as adjuvant treatment for stage III colon cancer. New Engl. J. Med. 352, 2696–2704 (2005).
Kuderer, N. M., Dale, D. C., Crawford, J., Cosler, L. E. & Lyman, G. H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106, 2258–2266 (2006).
Ahmed, G. et al. Cost implications of reactive versus prospective testing for dihydropyrimidine dehydrogenase (DPD) deficiency in patients with colorectal cancer. J. Clin. Oncol. 31, (Suppl.; Abstr 3627) (2013).
Blay, J. Y. et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J. Clin. Oncol. 14, 636–643 (1996).
Crawford, J., Dale, D. C. & Lyman, G. H. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100, 228–237 (2004).
Acknowledgements
Core funding to the Wellcome Trust Centre for Human Genetics was provided by the Wellcome Trust (090532/Z/09/Z).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.K. is a co-founder of and owns stock in Oxford Cancer Biomarkers, Oxford, UK. R.K. owns stock in Oxford Cancer Biomarkers. K.M. is an employee at Oxford Cancer Biomarkers. I.T. is a consultant for Oxford Cancer Biomarkers. All other authors declare no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Church, D., Kerr, R., Domingo, E. et al. 'Toxgnostics': an unmet need in cancer medicine. Nat Rev Cancer 14, 440–445 (2014). https://doi.org/10.1038/nrc3729
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3729
This article is cited by
-
Hydrogen-bonded organic framework-based bioorthogonal catalysis prevents drug metabolic inactivation
Nature Catalysis (2023)
-
Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model
Scientific Reports (2021)
-
Molecular and Functional Diagnostic Tools in Precision Oncology for Urological Malignancies
Indian Journal of Surgical Oncology (2017)
-
HUS1 regulates in vivo responses to genotoxic chemotherapies
Oncogene (2016)
-
Use of semantic workflows to enhance transparency and reproducibility in clinical omics
Genome Medicine (2015)