Abstract
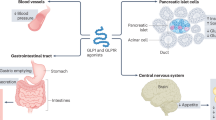
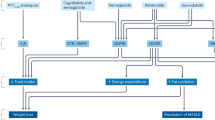
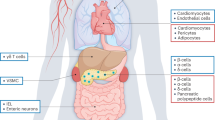
Glucagon-like peptide 1 (GLP-1) is an incretin hormone responsible for amplification of insulin secretion when nutrients are given orally, as opposed to intravenously, and it retains its insulinotropic activity in patients with type 2 diabetes mellitus. GLP-1-based therapies, such as GLP-1 receptor agonists and inhibitors of dipeptidyl peptidase 4, an enzyme that degrades endogenous GLP-1, have established effectiveness in lowering glucose levels and are routinely used to treat patients with type 2 diabetes. These agents regulate glucose metabolism through multiple mechanisms and have several effects on cardiovascular parameters. These effects, possibly independent of the glucose-lowering activity, include changes in blood pressure, endothelial function, body weight, cardiac metabolism, lipid metabolism, left ventricular function, atherosclerosis, and the response to ischemia–reperfusion injury. Thus, GLP-1-based therapies could potentially target both diabetes and cardiovascular disease. This Review highlights the mechanisms targeted by GLP-1-based therapies, and emphasizes current developments in incretin research that are relevant to cardiovascular risk and disease, as well as treatment with GLP-1 receptor agonists.
Key Points
-
Glucagon-like peptide 1 receptor (GLP-1R) agonists lower blood pressure in patients with type 2 diabetes mellitus
-
GLP-1R agonists have a long-term, weight-reducing effect in the majority of patients with type 2 diabetes
-
Ischemic preconditioning and postconditioning with GLP-1R agonists results in a reduction of ≤50% in the size of myocardial infarctions in animal models, and inhibit myocardial stunning and dysfunction in humans
-
GLP-1R agonists reduce the levels of biomarkers that have been linked to atherosclerotic cardiovascular disease and, therefore, might inhibit atherosclerosis development
-
GLP-1R agonists might improve endothelial dysfunction
-
To date, clinical studies of GLP-1R agonists have shown improvement, rather than impairment, in cardiovascular parameters
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas 4th edn (International Diabetes Federation, Brussels, 2009).
Wild, S., Roglic, G., Green, A., Sicree, R. & King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053 (2004).
Mata-Cases, M. et al. Incidence of complications and mortality in a type 2 diabetes patient cohort study followed up from diagnosis in a primary healthcare center. Int. J. Clin. Pract. 65, 299–307 (2011).
Haffner, S. M., Lehto, S., Ronnemaa, T., Pyorala, K. & Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234 (1998).
Juutilainen, A., Lehto, S., Ronnemaa, T., Pyorala, K. & Laakso, M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 28, 2901–2907 (2005).
Nauck, M. A. et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 63, 492–498 (1986).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Holst, J. J., Madsbad, S. & Schmitz, O. in Textbook of Diabetes 4th edn (eds Holt, R. I. G., Cockram, C. S., Flyvbjerg, A & Goldstein, B) 478–493 (Wiley–Blackwell Publishing, New Jersey, (2010).
Deacon, C. F. et al. Dipeptidyl peptidase IV resistant analogs of glucagon-like peptide 1 which have extended metabolic stability and improved biological activity. Diabetologia 41, 271–278 (1998).
Deacon, C. F. et al. Both subcutaneously and intravenously administered glucagon-like peptide 1 are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44, 1126–1131 (1995).
Bose, A. K., Mocanu, M. M., Carr, R. D., Brand, C. L. & Yellon, D. M. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54, 146–151 (2005).
Nauck, M. A. et al. Preserved incretin effect in type 1 diabetic patients with end-stage nephropathy treated by combined heterotopic pancreas and kidney transplantation. Acta Diabetol. 30, 39–45 (1993).
Vilsbøll, T., Krarup, T., Madsbad, S. & Holst, J. J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 45, 1111–1119 (2002).
Vilsbøll, T., Agersø, H., Krarup, T. & Holst, J. J. Similar elimination rates of glucagon-like peptide 1 in obese type 2 diabetic patients and healthy subjects. J. Clin. Endocrinol. Metab. 88, 220–224 (2003).
Mayo, K. E. et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 55, 167–194 (2003).
Fehmann, H. C. et al. Ligand-specificity of the rat GLP-I receptor recombinantly expressed in Chinese hamster ovary (CHO-) cells. Z. Gastroenterol. 32, 203–207 (1994).
Alvarez, E. et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 92, 798–806 (2005).
Ban, K. et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 (2008).
Bullock, B. P., Heller, R. S. & Habener, J. F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137, 2968–2978 (1996).
Campos, R. V., Lee, Y. C. & Drucker, D. J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134, 2156–2164 (1994).
Egan, J. M., Montrose-Rafizadeh, C., Wang, Y., Bernier, M. & Roth, J. Glucagon-like peptide-1(7–36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology 135, 2070–2075 (1994).
Kanse, S. M., Kreymann, B., Ghatei, M. A. & Bloom, S. R. Identification and characterization of glucagon-like peptide 1 7–36 amide-binding sites in the rat brain and lung. FEBS Lett. 241, 209–212 (1988).
Korner, M., Stockli, M., Waser, B. & Reubi, J. C. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J. Nucl. Med. 48, 736–743 (2007).
Lankat-Buttgereit, B., Goke, R., Fehmann, H. C., Richter, G. & Goke, B. Molecular cloning of a cDNA encoding for the GLP-1 receptor expressed in rat lung. Exp. Clin. Endocrinol. 102, 341–347 (1994).
Larsen, P. J., Tang-Christensen, M., Holst, J. J. & Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270 (1997).
Merchenthaler, I., Lane, M. & Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403, 261–280 (1999).
Orskov, C. & Poulsen, S. S. Glucagonlike peptide-I-(7–36)-amide receptors only in islets of Langerhans. Autoradiographic survey of extracerebral tissues in rats. Diabetes 40, 1292–1296 (1991).
Richter, G. et al. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am. J. Physiol. 265, L374–L381 (1993).
Satoh, F. et al. Characterization of human and rat glucagon-like peptide-1 receptors in the neurointermediate lobe: lack of coupling to either stimulation or inhibition of adenylyl cyclase. Endocrinology 141, 1301–1309 (2000).
Shimizu, I., Hirota, M., Ohboshi, C. & Shima, K. Identification and localization of glucagon-like peptide 1 and its receptor in rat brain. Endocrinology 121, 1076–1082 (1987).
Uttenthal, L. O. & Blazquez, E. Characterization of high-affinity receptors for truncated glucagon-like peptide-1 in rat gastric glands. FEBS Lett. 262, 139–141 (1990).
Uttenthal, L. O., Toledano, A. & Blazquez, E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7–36) amide in rat brain. Neuropeptides 21, 143–146 (1992).
Wei, Y. & Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 358, 219–224 (1995).
Arnes, L., Moreno, P., Nuche-Berenguer, B., Valverde, I. & Villanueva-Penacarrillo, M. L. Effect of exendin-4 treatment upon glucose uptake parameters in rat liver and muscle, in normal and type 2 diabetic state. Regul. Pept. 153, 88–92 (2009).
Green, B. D. et al. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch. Biochem. Biophys. 478, 136–142 (2008).
Bjerre Knudsen, L. et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151, 1473–1486 (2010).
FDA Briefing Materials Table of Contents—Liraglutide. FDA Website [online] (2011).
Hegedüs, L., Moses, AC, Zdravkovic, M, Le Thi, T. & Daniels, GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J. Clin. Endocrinol. Metab. 96, 853–860 (2011).
Nystrom, T. et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 287, E1209–E1215 (2004).
Sonne, D. P., Engstrom, T. & Treiman, M. Protective effects of GLP-1 analogs exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept 146, 243–249 (2008).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Jurado, J. et al. Prevalence of cardiovascular disease and risk factors in a type 2 diabetic population of the North Catalonia diabetes study. J. Am. Acad. Nurse. Pract. 21, 140–148 (2009).
Suwaidi, J. A. et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101, 948–954 (2000).
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317, 703–713 (1998).
Gutzwiller, J. P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004).
Astrup, A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. (Lond) http://dx.doi.org/10.1038/ijo.2011.158.
Basu, A. et al. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am. J. Physiol. Endocrinol. Metab. 293, E1289–E1295 (2007).
Golpon, H. A., Puechner, A., Welte, T., Wichert, P. V. & Feddersen, C. O. Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul. Pept. 102, 81–86 (2001).
Nystrom, T., Gonon, A. T., Sjoholm, A. & Pernow, J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 125, 173–177 (2005).
Ozyazgan, S., Kutluata, N., Afsar, S., Ozdas, S. B. & Akkan, A. G. Effect of glucagon-like peptide-1(7–36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology 74, 119–126 (2005).
Hansen, L., Hartmann, B., Mineo, H. & Holst, J. J. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul. Pept. 118, 11–18 (2004).
Barragan, J. M., Rodriguez, R. E. & Blazquez, E. Changes in arterial blood pressure and heart rate induced by glucagon- like peptide-1-(7–36) amide in rats. Am. J. Physiol. 266, E459–E466 (1994).
Barragan, J. M., Rodriguez, R. E., Eng, J. & Blazquez, E. Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul. Pept. 67, 63–68 (1996).
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Bucinskaite, V. et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil. 21, 978–e78 (2009).
Nishizawa, M. et al. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J. Auton. Nerv. Syst. 80, 14–21 (2000).
Barragan, J. M., Eng, J., Rodriguez, R. & Blazquez, E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am. J. Physiol. 277, E784–E791 (1999).
Bojanowska, E. & Stempniak, B. Effects of centrally or systemically injected glucagon-like peptide-1 (7–36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul. Pept. 91, 75–81 (2000).
Isbil-Buyukcoskun, N. & Gulec, G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul. Pept. 118, 33–38 (2004).
Yamamoto, H. et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Invest. 110, 43–52 (2002).
Yu, M. et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens. 21, 1125–1135 (2003).
Moreno, C., Mistry, M. & Roman, R. J. Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 434, 163–167 (2002).
Toft-Nielsen, M. B., Madsbad, S. & Holst, J. J. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22, 1137–1143 (1999).
Halbirk, M. et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am. J. Physiol. Heart Circ. Physiol. 298, H1096–H1102 (2010).
Gutzwiller, J. P. et al. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 73, 142–150 (2006).
Okerson, T., Yan, P., Stonehouse, A. & Brodows, R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am. J. Hypertens. 23, 334–339 (2010).
Bergenstal, R. M. et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 376, 431–439 (2010).
Diamant, M. et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 375, 2234–2243 (2010).
Drucker, D. J. et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Buse, J. B. et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374, 39–47 (2009).
Garber, A. et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373, 473–481 (2009).
Marre, M. et al. Liraglutide, a once-daily human GLP-1 analog, added to a sulfonylurea over 26 weeks produces greater improvements in glycemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet. Med. 26, 268–278 (2009).
Nauck, M. et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 32, 84–90 (2009).
Russell-Jones, D. et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 52, 2046–2055 (2009).
Zinman, B. et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 32, 1224–1230 (2009).
Diaz, A., Bourassa, M. G., Guertin, M. C. & Tardif, J. C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur. Heart J. 26, 967–974 (2005).
Jensen, M. T., Marott, J. L., Allin, K. H., Nordestgaard, B. G. & Jensen, G. B. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: The Copenhagen City Heart Study. Eur. J. Cardiovasc. Prev. Rehabil. http://dx.doi.org/10.1177/1741826710394274.
Jouven, X. et al. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 352, 1951–1958 (2005).
Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Hattori, Y. et al. A glucagon-like peptide-1 (GLP-1) analog, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 53, 2256–2263 (2010).
Arakawa, M. et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 59, 1030–1037 (2010).
Hirano, T. et al. Incretins directly suppress the development of macrophage-driven atherosclerosis in apolipoprotein E-null mice. Diabetologia 53 (Supl. 1), S72 (2010).
Nagashima, M. et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 54, 2649–2659 (2011).
Liu, H., Dear, A. E., Knudsen, L. B. & Simpson, R. W. A long-acting glucagon-like peptide-1 analog attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J. Endocrinol. 201, 59–66 (2009).
Plutzky, J., Garber, A., Falahati, A., Toft, A. D. & Poulter, N. R. The once-daily human GLP-1 analogue, liraglutide, significantly reduces markers of cardiovascular risk in type 2 diabetes: a meta-analysis of six clinical trials [abstract]. Eur. Heart J. 30 (Suppl. 1), 917 (2009).
Viswanathan, P. et al. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr. Pract. 13, 444–450 (2007).
Courreges, J. P. et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analog, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet. Med. 25, 1129–1131 (2008).
Bunck, M. C. et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 33, 1734–1737 (2010).
Diamant, M. et al. Impact of exenatide once weekly and insulin glargine on glucose control and cardiovascular risk f actors in subjects with type 2 diabetes [abstract]. Diabetologia 53 (Suppl. 1), S344 (2010).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Bose, A. K., Mocanu, M. M., Carr, R. D. & Yellon, D. M. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc. Drugs Ther. 19, 9–11 (2005).
Bose, A. K., Mocanu, M. M., Carr, R. D. & Yellon, D. M. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc. Drugs Ther. 21, 253–256 (2007).
Ossum, A., van Deurs, U., Engstrom, T., Jensen, J. S. & Treiman, M. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9–36)a in an isolated rat heart. Pharmacol. Res. 60, 411–417 (2009).
Sager, P. et al. Exenatide once weekly did not affect the QTc interval in patients with type 2 diabetes. Presented at the 47th Annual Meeting of the European Association for the Study of Diabetes.
Timmers, L. et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J. Am. Coll. Cardiol. 53, 501–510 (2009).
Kristensen, J. et al. Lack of cardioprotection from subcutaneously and preischemic administered liraglutide in a closed chest porcine ischemia reperfusion model. BMC Cardiovasc. Disord. 9, 31 (2009).
Noyan-Ashraf, M. H. et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58, 975–983 (2009).
Nikolaidis, L. A. et al. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J. Pharmacol. Exp. Ther. 312, 303–308 (2005).
Nikolaidis, LA. et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109, 962–965 (2004).
Read, P. A. et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ. Cardiovasc. Interv. 4, 266–272 (2011).
Lønborg, J. et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehr309.
Ravassa, S., Zudaire, A., Carr, R. D. & Diez, J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 300, H1361–H1372 (2011).
Kavianipour, M. et al. Glucagon-like peptide-1 (7–36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides 24, 569–578 (2003).
Vila Petroff, M. G., Egan, J. M., Wang, X. & Sollott, S. J. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ. Res. 89, 445–452 (2001).
Bhashyam, S. et al. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ. Heart Fail. 3, 512–521 (2010).
Nikolaidis, L. A. et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110, 955–961 (2004).
Nikolaidis, L. A., Elahi, D., Shen, Y. T. & Shannon, R. P. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 289, H2401–H2408 (2005).
Zhao, T. et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 317, 1106–1113 (2006).
Nguyen, T. D. Oleate controls the effects of GLP-1 and exendin-4 on myocardial glucose utilization and contractile function [abstract]. Eur. Heart J. 37 (Suppl. 1), 937 (2010).
Nathanson, D. et al. Plasma levels of glucagon like peptide-1 associate with diastolic function in elderly men. Diabet. Med. 28, 301–305 (2011).
Sokos, G. G., Nikolaidis, L. A., Mankad, S., Elahi, D. & Shannon, R. P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Fail. 12, 694–699 (2006).
Mussig, K. et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am. J. Cardiol. 102, 646–647 (2008).
Heller, S. R. Abnormalities of the electrocardiogram during hypoglycemia: the cause of the dead in bed syndrome? Int. J. Clin. Pract. Suppl. 129, 27–32 (2002).
Linnebjerg, H. et al. A thorough QT study to evaluate the effects of singledose exenatide 10 mug on cardiac repolarization in healthy subjects. Int. J. Clin. Pharmacol. Ther. 49, 594–604 (2011).
Chatterjee, D. J., Khutoryansky, N., Zdravkovic, M., Sprenger, C. R. & Litwin, J. S. Absence of QTc prolongation in a thorough QT study with subcutaneous liraglutide, a once-daily human GLP-1 analog for treatment of type 2 diabetes. J. Clin. Pharmacol. 49, 1353–1362 (2009).
Sokos, G. G. et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am. J. Cardiol. 100, 824–829 (2007).
Williamson, D. F. et al. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 23, 1499–1504 (2000).
Klonoff, D. C. et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 24, 275–286 (2008).
Moretto, T. J. et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin. Ther. 30, 1448–1460 (2008).
Jendle, J. et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analog for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes. Metab. 11, 1163–1172 (2009).
Hsieh, J. et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 53, 552–561 (2010).
Schwartz, E. A. et al. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 212, 217–222 (2010).
Dokken, B. B. et al. Glucagon-like peptide-1 (GLP-1) attenuates post-resuscitation myocardial microcirculatory dysfunction. Resuscitation 81, 755–760 (2010).
Ku, H. C., Chen, W. P. & Su, M. J. GLP-1 signaling preserves cardiac function in endotoxemic Fischer 344 and DPP4-deficient rats. Naunyn-Schmiedebergs Archives of Pharmacology 382, 463–474 (2010).
Nissen, S. E. & Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 (2007).
Rao, A. D., Kuhadiya, N., Reynolds, K. & Fonseca, V. A. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: a meta-analysis of observational studies. Diabetes Care 31, 1672–1678 (2008).
Yudkin, J. S., Lehman, R. & Krumholz, H. M. Glucagon-like peptide-1 drugs. Use of GLP-1 analogs needs great caution. BMJ 342, d1478 (2011).
Best, J. H. et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care 34, 90–95 (2011).
Blevins, T. et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 96, 1301–1310 (2011).
Buse, J. B. et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27, 2628–2635 (2004).
DeFronzo, R. A. et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28, 1092–1100 (2005).
Kendall, D. M. et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28, 1083–1091 (2005).
Barakat, G. M. et al. Role of glucagon-like peptide-1 and its agonists on early prevention of cardiac remodeling in type 1 diabetic rat hearts. Gen. Physiol. Biophys. 30, 34–44 (2011).
Nakagawa, A. et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci. 110, 36–43 (2004).
Delgado, E. et al. Glucagon-like peptide-1 binding to rat skeletal muscle. Peptides 16, 225–229 (1995).
Author information
Authors and Affiliations
Contributions
J. Sivertsen, T. Vilsbøll and J. J. Holst contributed to all aspects of the article, including researching data, discussion of content, and writing, reviewing, and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. Sivertsen declares no competing interests. J. Rosenmeier declares that she has received grant support from Merck Sharp & Dohme. J. J. Holst declares that he has been a consultant for GlaxoSmithKline, Novo Nordisk, and Zealand Pharmaceuticals. He has also received grants from Merck Sharp & Dohme and Novartis. T. Vilsbøll declares that she has been a consultant for and received honoraria from Amylin, Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. She has also received honoraria from Novartis, and grant support from Merck Sharp & Dohme.
Rights and permissions
About this article
Cite this article
Sivertsen, J., Rosenmeier, J., Holst, J. et al. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 9, 209–222 (2012). https://doi.org/10.1038/nrcardio.2011.211
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.211
This article is cited by
-
Liraglutide to Improve corONary haemodynamics during Exercise streSS (LIONESS): a double-blind randomised placebo-controlled crossover trial
Diabetology & Metabolic Syndrome (2021)
-
Dipeptidyl-peptidase-4 (DPP-4) inhibitor ameliorates 5-flurouracil induced intestinal mucositis
BMC Cancer (2019)
-
Glucagon-like peptide 1 in health and disease
Nature Reviews Endocrinology (2018)
-
Antidiabetic activity of mefloquine via GLP-1 receptor modulation against STZ–NA-induced diabetes in albino wistar rats
3 Biotech (2018)
-
Clinical Impact of 5 Years of Liraglutide Treatment on Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus in a Real-Life Setting in Italy: An Observational Study
Diabetes Therapy (2018)