Abstract
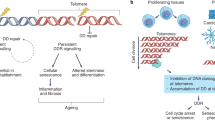
Cellular senescence, defined as arrest during the cell cycle (G0), is involved in the complex process of the biological ageing of tissues, organs, and organisms. Senescence is driven by many factors including oxidative stress, the DNA damage and repair response, inflammation, mitogenic signals, and telomere shortening. Telomeres are shortened by each cell division until a critical length is reached and dysfunction ensues. DNA-repair pathways are then recruited and cells enter senescence, losing their capacity to proliferate. In addition to cell division, factors causing telomere shortening include DNA damage, inflammation, and oxidative stress. Both cardiovascular risk factors and common cardiovascular diseases, such as atherosclerosis, heart failure, and hypertension, are associated with short leucocyte telomeres, but causality remains undetermined. Telomere length does not satisfy strict criteria for being a biomarker of ageing, but adds predictive power to that of chronological age, and can be considered a marker of cardiovascular ageing. The 'senescence-associated secretory phenotype' of senescent cells exerts a wide range of autocrine and paracrine activities aimed at tissue repair, but which also fuel degenerative and proliferative alterations that contribute to cardiovascular disease. In this Review, the relationship between telomere shortening, senescence, and cardiovascular disease is discussed.
Key Points
-
Cellular senescence—arrest during the cell cycle (G0)—is involved in the ageing process, and driven by oxidative stress, DNA damage and repair response, inflammation, mitogenic signals, and telomere shortening
-
Cellular senescence parallels the development of atherosclerosis and other pathologies in the vasculature and heart and is, therefore, likely to have a pivotal role in cardiovascular disease
-
Telomere length, usually measured as leucocyte telomere length, is widely considered a marker of biological ageing, is largely inherited, and is modulated by various intrinsic and environmental factors throughout life
-
Endogenous factors causing telomere attrition include ageing, cell division, genetic factors, DNA damage, inflammation, and oxidative stress; telomere attrition can be retarded by genetic factors, telomerase, oestrogen, and antioxidants
-
Environmental factors associated with telomere shortening include poor lifestyle (smoking, excess calories, sedentary lifestyle, alcohol abuse), and severe mental stress, whereas healthy lifestyle is associated with maintenance of long telomeres
-
Telomere length seems to have a key role in cardiovascular disease by driving cells into cell-cycle arrest, senescence, and ultimately apoptosis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Campisi, J., Andersen, J. K., Kapahi, P. & Melov, S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin. Cancer Biol. 21, 354–359 (2011).
Kovacic, J. C., Moreno, P., Hachinski, V., Nabel, E. G. & Fuster, V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation 123, 1650–1660 (2011).
Kovacic, J. C., Moreno, P., Nabel, E. G., Hachinski, V. & Fuster, V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review. Circulation 123, 1990–1910 (2011).
Zhu, H., Belcher, M. & van der Harst, P. Healthy aging and disease: a role for telomere biology? Clin. Sci. 120, 427–440 (2011).
Wang, J. C. & Bennett, M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 111, 245–259 (2012).
Mather, K. A., Jorm, A. F., Parslow, R. A. & Christensen, H. Is telomere length a biomarker of aging? A review. J. Gerontol. A Biol. Sci. Med. Sci. 66, 202–213 (2011).
Der, G. et al. Is telomere length a biomarker of aging: cross-sectional evidence from the west of Scotland? PLoS ONE 7, e45166 (2012).
Samani, N. J., Boultby, R., Butler, R., Thompson, J. R. & Goodall, A. H. Telomere shortening in atherosclerosis. Lancet 358, 472–473 (2001).
Samani, N. J. & van der Harst, P. Biological ageing and cardiovascular disease. Heart 94, 537–539 (2008).
Blasco, M. A. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 6, 611–622 (2005).
Calado, R. T. & Young, N. S. Telomere diseases. N. Engl. J. Med. 361, 2353–2365 (2009).
von Zglinicki, T., Saretzki, G., Ladhoff, J., d' Adda di Fagagna, F. & Jackson, S. P. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 126, 111–117 (2005).
Campisi, J. Cellular senescence: putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 21, 107–112 (2011).
Rodier, F. et al. DNA-SCARS: Distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokines. J. Cell. Sci. 124, 68–81 (2011).
Fumagalli, M. et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355–365 (2012).
Minamino, T. et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002).
Erusalimsky, J. D. & Kurz, D. J. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp. Gerontol. 40, 634–642 (2005).
Matthews, G. et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 99, 156–164 (2006).
Chang, E. & Harley, C. B. Telomere length and replicative aging in human vascular tissues. Proc. Natl Acad. Sci. USA 92, 11190–11194 (1995).
Okuda, K. et al. Telomere shortening in human abdominal aorta: relationship with age and atherosclerosis. Atherosclerosis 152, 391–398 (2000).
Aviv, H. et al. Age dependent aneuploidy and telomere length of the human endothelium. Atherosclerosis 159, 281–287 (2001).
Benetos, A. et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 43, 182–185 (2004).
Huzen, J. et al. Circulating leukocyte and carotid atherosclerotic plaque telomere length: interrelation, association with plaque characteristics, and restenosis after endarterectomy. Arterioscler. Thromb. Vasc. Biol. 31, 1219–1225 (2011).
Nzietchueng, R. et al. Telomere length in vascular tissues from patients with atherosclerotic disease. J. Nutr. Health Aging 15, 153–156 (2011).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Hoffmann, J. et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ. Res. 89, 709–715 (2001).
El Assar, M. et al. Mechanisms involved in the aging-induced vascular dysfunction. Front. Physiol. 3, 132 (2012).
Blackburn, E. H. Switching and signaling at the telomere. Cell 106, 661–667 (2001).
Blackburn, E. H., Greider, C. W. & Szostak, J. W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 12, 1133–1138 (2006).
Blasco, M. A. Telomere length, stem cells and aging. Nat. Chem. Biol. 3, 640–649 (2007).
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2001–2010 (2005).
Price, C. M. et al. Evolution of CST function and telomere maintenance. Cell Cycle 9, 3157–3165 (2010).
Levy, D. et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc. Natl Acad. Sci. USA 107, 9293–9298 (2010).
Mangino, M. et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum. Mol. Genet. 21, 5385–5394 (2012).
Cesare, A. J. & Reddel, R. R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat. Rev. Genet. 11, 319–330 (2010).
Shay, J. W. & Wright, W. E. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 26, 867–874 (2005).
Karlseder, J., Smogorzewska, A. & de Lange, T. Senescence induced by altered telomere state, not telomere loss. Science 295, 2446–2449 (2002).
Cesare, A. J. & Karlseder, J. A. Three-state model of telomere control over human proliferative boundaries. Curr. Opin. Cell. Biol. 24, 731–738 (2012).
Sarin, K. Y. et al. Conditional telomerase induction causes proliferation of hair follicle cells. Nature 436, 1048–1052 (2005).
Rao, T. P. & Kühl, M. An updated overview on Wnt signaling pathways: a prelude for more. Circ. Res. 106, 1798–1806 (2010).
Savage, S. A. et al. Genetic variation, nucleotide diversity, and linkage disequilibrium in seven telomere stability genes suggest that these genes may be under constraint. Hum. Mutat. 26, 343–350 (2005).
Jacobs, T. L. et al. Intensive mediation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology 36, 664–681 (2011).
Blasco, M. A. The epigenetic regulation of mammalian telomeres. Nat. Rev. Genet. 8, 299–309 (2007).
Aviv, A. et al. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 39, e134 (2011).
Hoffmann, J. & Spyridopoulos, I. Telomere length in cardiovascular disease: new challenges in measuring this marker of cardiovascular aging. Future Cardiol. 6, 789–803 (2011).
Kimura, M. et al. Telomere length and mortality in elderly Danish twins. Am. J. Epidemiol. 167, 799–806 (2008).
Fyhrquist, F. et al. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J. Intern. Med. 267, 278–286 (2010).
Hemann, M. T. et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107, 67–77 (2001).
Zou, Y. et al. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol. Biol. Cell 15, 3709–3718 (2004).
Vera, E. & Blasco, M. A. Beyond average; potential for measurement of short telomeres. Aging (Albany NY) 4, 379–392 (2012).
Butler, M. G. et al. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet. Cytogenet. 105, 138–144 (1998).
Aviv, A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat. Res. 730, 68–74 (2012).
Mangino, M. et al. A genome-wide association study identifies a novel locus on chromosome 18q12.2 influencing cell telomere length. J. Med. Genet. 46, 451–454 (2009).
Codd, V. et al. Common variants near TERC are associated with mean telomere length. Nat. Genet. 42, 197–199 (2010).
Kirwan, M. & Dokal, I. Dyskeratosis congenita, stem cells and telomeres. Biochim. Biophys. Acta 1792, 371–379 (2009).
Armanios, M. & Blackburn E. H. The telomere syndromes. Nat. Rev. Genet. 13, 693–704 (2012).
Guarante, L. The Franklin H. Epstein lecture: sirtuins, aging, and medicine. N. Engl. J. Med. 364, 2235–2244 (2011).
Kim, S. et al. Telomere maintenance genes SIRT1 and XRCC6 impact age-related decline in telomere length but only SIRT1 is associated with human longevity. Biogerontology 13, 119–131 (2012).
Fyhrquist, F. et al. Telomere length is associated with ACE I/D polymorphism in hypertensive patients with left ventricular hypertrophy. J. Renin Angiotensin Aldosterone Syst. http://dx.doi.org/10.1177/1470320312460292.
Vasan, R. S. et al. Association of leukocyte telomere length with circulating biomarkers of the renin–angiotensin—aldosterone system: the Framingham Heart Study. Circulation 117, 1138–1144 (2008).
Sidorov, I., Kimura, M., Yashin, A. & Aviv, A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp. Hematol. 37, 514–524 (2009).
Akkad, A. et al. Telomere length in small-for-gestational-age babies. BJOG 113, 318–323 (2006).
Raqib, R. et al. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am. J. Clin. Nutr. 85, 845–852 (2007).
Kajantie, E. et al. No association between body size at birth and leucocyte telomere length in adult life—evidence from three cohort studies. Int. J. Epidemiol. 41, 1400–1408 (2012).
Okuda, K. et al. Telomere length in the newborn. Pediatr. Res. 52, 377–381 (2002).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
Bekaert, S. et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell 6, 639–647 (2007).
Strandberg, T. E. et al. Telomere length in old age and cholesterol across the life course. J. Amer. Geriatr. Soc. 59, 1979–1981 (2011).
Kyo, S. et al. Estrogen activates telomerase. Cancer Res. 59, 5917–5921 (1999).
Valdes, A. M. et al. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664 (2005).
Strandberg, T. E. et al. Association of telomere length in older men with mortality and midlife body mass index and smoking. J. Gerontol. A Biol. Sci. Med. Sci. 66, 815–820 (2011).
Carnevali, S. et al. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L955–L963 (2003).
Farhat, N. et al. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can. J. Physiol. Pharmacol. 86, 761–769 (2008).
Strandberg, T. E. et al. Association between alcohol consumption in healthy midlife and telomere length in older men: the Helsinki Businessmen Study. Eur. J. Epidemiol. 27, 815–822 (2012).
Comporti, M. et al. Ethanol-induced oxidative stress: basic knowledge. Genes Nutr. 5, 101–109 (2010).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17312–17315 (2004).
Wolkowitz, O. M. et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS ONE 6, e17837 (2011).
Kananen, L. et al. Childhood adversities are associated with shorter telomeres at adult age both in individuals with an anxiety disorder and controls. PLoS ONE 5, 10826 (2010).
Lin, J., Epel, E. & Blackburn, E. Telomeres and lifestyle factors: roles in cellular aging. Mutat. Res. 730, 85–89 (2012).
Farzaneh-Far, R. et al. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 303, 250–257 (2010).
Richards, B. J. et al. Higher vitamin D concentrations are associated with longer leukocyte telomere length in women. Am. J. Clin. Nutr. 86, 1420–1425 (2007).
Xu, Q. et al. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 89, 1857–1863 (2009).
Cherkas, L. F. et al. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 168, 154–158 (2008).
Werner, C. et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120, 2438–2447 (2009).
Njajou, O. T. et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 64, 860–864 (2009).
Krauss, J. et al. Physical fitness and telomere length in patients with coronary heart disease: findings from the Heart and Soul Study. PLoS ONE 6, e26983 (2011).
Ornish, D. et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 9, 1048–1057 (2008).
Brouilette, S. W. et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369, 107–114 (2007).
Rando, T. A. The immortal strand hypothesis: segregation and construction. Cell 129, 1239–1243 (2007).
Flores, I., Benetti, R. & Blasco M. A. Telomerase regulation and stem cell behavior. Curr. Opin. Cell Biol. 18, 254–260 (2006).
Kimura, M. et al. Synchrony of telomere length among hematopoietic stem cells. Exp. Hematol. 38, 854–859 (2010).
Oeseburg, H. et al. Can critically short telomeres cause functional exhaustion of progenitor cells in postinfarction heart failure? J. Am. Coll. Cardiol. 50, 1911–1912 (2007).
Spyridopoulos, I. et al. Telomere gap between granulocytes and lymphocytes is a determinant for hematopoietic preogenitor cell impairment in patients with previous myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 28, 968–974 (2008).
Chimenti, C. et al. Senescence and death of primitive cells and myocytes lead premature cardiac aging and heart failure. Circ. Res. 93, 604–613 (2003).
Anversa, P., Kajstura, J., Leri, A. & Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation 113, 1451–1463 (2006).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Svenson, U. et al. Blood cell telomere length is a dynamic feature. PLoS ONE 6, e21485 (2011).
Farzaneh-Far, R. et al. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the Heart and Soul Study. PLoS ONE 5, e8612 (2010).
Wong, L. S. et al. Telomere biology in cardiovascular disease: the TERC-/- mouse as a model for heart failure and ageing. Cardiovasc. Res. 81, 244–252 (2009).
Breslow, J. L. Mouse models of atherosclerosis. Science 272, 685–688 (1996).
Zadelaar, S. et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 27, 1706–1721 (2007).
Poch, E. et al. Short telomeres protect from diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB J. 18, 418–420 (2004).
Leri, A. et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 22, 131–139 (2003).
Minamino, T., Miyauchi, H., Yoshida, T., Tateno, K. & Komuro, I. The role of vascular cell senescense in atherosclerosis: antisenescense as a novel therapeutic strategy for vascular aging. Curr. Vasc. Pharmacol. 2, 141–148 (2004).
Minamino, T. & Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 100, 15–26 (2007).
Hastings, R., Qureshi, M., Verma, R., Lacy, P. S. & Williams, B. Telomere attrition and accumulation of senescent cells in cultured human endothelial cells. Cell Prolif. 37, 317–324 (2004).
Matthews, C. et al. Vascular smooth muscle cells undergo telomere-based senescense in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 99, 156–164 (2006).
Leri, A., Malhotra, A., Liew, C. C., Kajstura, J. & Anversa, P. Telomerase activity in rat cardiac myocytes is age and gender dependent. J. Mol. Cell. Cardiol. 32, 385–390 (2000).
Kajstura, J. et al. The telomere–telomerase axis and the heart. Antioxid. Redox Signal. 8, 2125–2141 (2006).
Kajstura, J. et al. Cardiomyogenesis in the aging and failing human heart. Circulation 126, 1869–1881 (2012).
Rosenzweig, A. Cardiac regeneration. Science 338, 1549–1550 (2012).
Torella, A. et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ. Res. 94, 514–524 (2004).
van der Harst, P. et al. Telomere length of circulating leukocytes is decreased with chronic heart failure. J. Am. Coll. Cardiol. 49, 1459–1464 (2007).
Moslehi, J. DePinho, R. A. & Sahin, E. Telomeres and mitochondria in the aging heart. Circ. Res. 110, 1226–1237 (2012).
Rajabi, M., Kassiotis, C., Razeghi, P. & Taegtmeyer, H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail. Rev. 12, 331–343 (2007).
Neubauer, S. The failing heart—an engine out of fuel. N. Engl. J. Med. 356, 1140–1151 (2007).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set-up” for vascular disease. Circulation 107, 139–146 (2003).
Lakatta, E. G. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 107, 490–497 (2003).
O'Donnell, C. J. et al. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 28, 1165–1171 (2008).
Brouilette, S., Singh, R. K., Thompson, J. R., Goodall, A. H. & Samani, N. J. White cell telomere length and risk of premature myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 23, 842–846 (2003).
Farzaneh-Far, R. et al. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler. Thromb. Vasc. Biol. 28, 1379–1384 (2008).
Jeanclos, E. et al. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 36, 195–200 (2000).
Fyhrquist, F. et al. Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy, the LIFE study. J. Hum. Hypertens. 25, 711–718 (2011).
Ding, H. et al. Telomere length and risk of stroke in Chinese. Stroke 43, 658–663 (2012).
Zee, R. Y. L., Castonguay, A. J., Barton N. S. & Ridker, P. M. Relative leukocyte telomere length and risk of incident ischemic stroke in men: a prospective, nested case-control approach. Rejuvenation Res. 13, 411–414 (2010).
Brouilette, S. W. et al. Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart 94, 422–425 (2008).
Wong, L. S. et al. Telomere length of circulating leukocyte subpopulations and buccal cells in patients with ischemic heart failure and their offspring. PLoS ONE 6, 23118 (2011).
De Meyer, T. et al. No shorter telomeres in subjects with a family history of cardiovascular disease in the Asklepios Study. Arterioscler. Thromb. Vasc. Biol. 32, 3076–3081 (2012).
Voghel, G. et al. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech. Ageing Dev. 128, 662–671 (2007).
Thorin, E. & Thorin-Trescases, N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovasc. Res. 84, 24–32 (2009).
Brandes, R. P., Fleming, I. & Busse, R. Endothelial aging. Cardiovasc. Res. 66, 286–294 (2005).
Voghel, G. et al. Endogenous oxidative stress prevents telomerase-dependent immortalization of human endothelial cells. Mech. Ageing Dev. 131, 354–363 (2010).
Imanishi, T., Hano, T. & Nishio, I. Angiotensin II accelerated endothelial progenitor cell senescence through induction of oxidative stress. J. Hypertens. 23, 97–104 (2005).
Feng, X, Wang, L. & Li, Y. Change of telomere length in angiotensin-induced human glomerular mesangial cell senescence and the protective role of losartan. Mol. Med. Report. 4, 255–260 (2011).
Spyridopoulos, I. et al. Statins enhance migratory capacity by upregulation of the telemere repeat-binding factor TR2 in endothelial progenitor cells. Circulation 110, 3136–3142 (2004).
Harley, C. B. Telomerase and cancer therapeutics. Nat. Rev. Cancer 8, 167–179 (2008).
Author information
Authors and Affiliations
Contributions
F. Fyhrquist and O. Saijonmaa researched data for the article, and all the authors contributed substantially to discussion of its content. F. Fyhrquist and O. Saijonmaa wrote the article, and all the authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fyhrquist, F., Saijonmaa, O. & Strandberg, T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol 10, 274–283 (2013). https://doi.org/10.1038/nrcardio.2013.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2013.30
This article is cited by
-
Persistent dyslipidemia increases the longitudinal changes in telomere length
Lipids in Health and Disease (2023)
-
Relationship between expression levels of TDRD7 and CRYBB3 and development of age-related cortico-nuclear cataracts
Egyptian Journal of Medical Human Genetics (2023)
-
The associations of socioeconomic status with incident dementia and Alzheimer’s disease are modified by leucocyte telomere length: a population-based cohort study
Scientific Reports (2023)
-
Telomere length dynamics measured by flow-FISH in patients with obesity undergoing bariatric surgery
Scientific Reports (2023)
-
Associations of measured and genetically predicted leukocyte telomere length with vascular phenotypes: a population-based study
GeroScience (2023)