Key Points
-
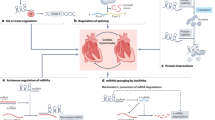
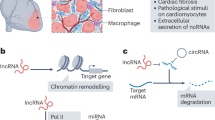
Cardiac fibrosis is an important event of the cardiac remodelling process, which includes activation of fibroblasts and exaggerated extracellular matrix production
-
Noncoding RNAs (ncRNAs) are important cellular regulators and divided into several classes, such as small microRNAs (∼22 nucleotides) and long noncoding RNAs (>200 nucleotides)
-
ncRNAs are detectable in blood and other body fluids and can be used for improved diagnosis of cardiac diseases including cardiac remodelling
-
ncRNAs provide powerful therapeutic targets that can be modulated in vivo by injection of specific oligonucleotides to repress cardiac diseases including the cardiac fibrosis process
-
Paracrine signalling and cell–cell interactions have a major role during cardiac remodelling, and ncRNAs can contribute to this process by active cellular secretion and uptake mechanisms within the cardiovascular environment
Abstract
Cardiac stress leads to remodelling of cardiac tissue, which often progresses to heart failure and death. Part of the remodelling process is the formation of fibrotic tissue, which is caused by exaggerated activity of cardiac fibroblasts leading to excessive extracellular matrix production within the myocardium. Noncoding RNAs (ncRNAs) are a diverse group of endogenous RNA-based molecules, which include short (∼22 nucleotides) microRNAs and long ncRNAs (of >200 nucleotides). These ncRNAs can regulate important functions in many cardiovascular cells types. This Review focuses on the role of ncRNAs in cardiac fibrosis; specifically, ncRNAs as therapeutic targets, factors for direct fibroblast transdifferentation, their use as diagnostic and prognostic markers, and their potential to function as paracrine modulators of cardiac fibrosis and remodelling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jessup, M. & Brozena, S. Heart failure. N. Engl. J. Med. 348, 2007–2018 (2003).
Hill, J. A. & Olson, E. N. Cardiac plasticity. N. Engl. J. Med. 358, 1370–1380 (2008).
Maron, B. J., & Maron, M. S. Hypertrophic cardiomyopathy. Lancet 381, 242–255 (2013).
Heusch, G. et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383, 1933–1943 (2014).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Gittenberger-de Groot, A. C. et al. The arterial and cardiac epicardium in development, disease and repair. Differentiation 84, 41–53 (2012).
Moore-Morris, T. et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Invest. 124, 2921–2934 (2014).
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Kumarswamy, R. et al. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 114, 1569–1575 (2014).
Kumarswamy, R. & Thum, T. Non-coding RNAs in cardiac remodeling and heart failure. Circ. Res. 113, 676–689 (2013).
Dangwal, S. & Thum, T. microRNA therapeutics in cardiovascular disease models. Annu. Rev. Pharmacol. Toxicol. 54, 185–203 (2014).
Rinn, J. L., Chang, H. Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81, 145–166 (2012).
Batista, P. J., Chang, H. Y. Long noncoding RNAs: cellular address codes in development and disease. Cell 152, 1298–1307 (2013).
Galindo, M. I., Pueyo, J. I., Fouix, S., Bishop, S. A. & Couso, J. P. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 5, e106 (2007).
Ørom, U. A. & Shiekhattar, R. Long coding RNAs usher in a new era in the biology of enhancers. Cell 154, 1190–1193 (2013).
Viereck, J., Bang, C., Foinquinos, A. & Thum, T. Regulatory RNAs and paracrine networks in the heart. Cardiovasc. Res. 102, 290–301 (2014).
Kumarswamy, R. et al. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler. Thromb. Vasc. Biol. 32, 361–369 (2012).
Volkmann, I. et al. MicroRNA-mediated epigenetic silencing of sirtuin1 contributes to impaired angiogenic responses. Circ. Res. 113, 997–1003 (2013).
Thum, T., Haverich, A., Borlak, J. Cellular dedifferentiation of endothelium is linked to activation and silencing of certain nuclear transcription factors: implications for endothelial dysfunction and vascular biology. FASEB J. 14, 740–751 (2000).
Ghosh, A. K., Nagpal, V., Covington, J. W., Michaels, M. A. & Vaughan, D. E. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): differential expression of microRNAs during EndMT. Cell. Signal. 24, 1031–1036 (2012).
van Rooij, E. et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316, 575–579 (2007).
da Costa Martins, P. A. et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 118, 1567–1576 (2008).
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 (2008).
Roy, S. et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 82, 21–29 (2009).
Liang, H. et al. A novel reciprocal loop between microRNA-21 and TGFβRIII is involved in cardiac fibrosis. Int. J. Biochem. Cell Biol. 44, 2152–2160 (2012).
Brønnum, H. et al. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving programmed cell death 4 and sprouty-1. PLoS ONE 8, e56280 (2013).
Villar, A. V., Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. Int. J. Cardiol. 167, 2875–2881 (2013).
Patrick, D. M. et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J. Clin. Invest. 120, 3912–3916 (2010).
Thum, T. et al. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J. Clin. Invest. 121, 461–462 (2011).
Liu, G. et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 (2010).
Chau, B. N. et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 4, 121ra18 (2012).
Queirós, A. M. et al. Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int. J. Cardiol. 169, 331–338 (2013).
Bang, C. et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 124, 2136–2146 (2014).
van Rooij, E. et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA 105, 13027–13032 (2008).
Boon, R. A. et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ. Res. 109, 1115–1119 (2011).
Melo, S. F. et al. Expression of microRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cell. Physiol. Biochem. 33, 657–669 (2014).
Zhu, J. N. et al. Smad3 inactivation and MiR-29b upregulation mediate the effect of carvedilol on attenuating the acute myocardium infarction-induced myocardial fibrosis in rat. PLoS ONE 8, e75557 (2013).
Abonnenc, M. et al. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ. Res. 113, 1138–1147 (2013).
Kumarswamy, R. et al. SERCA2a gene therapy restores microRNA-1 expression in heart failure via an Akt/FoxO3A-dependent pathway. Eur. Heart J. 33, 1067–1075 (2012).
Karakikes, I et al. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J. Am. Heart Assoc. 2, e000078 (2013).
Carè, A. et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 13, 613–618 (2007).
Matkovich, S. J. et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ. Res. 106, 166–175 (2010).
Chen, S. et al. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J. Cell. Mol. Med. 18, 415–421 (2014).
Castoldi, G. et al. MiR-133a regulates collagen 1A1: potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. J. Cell. Physiol. 227, 850–856 (2012).
Duisters, R. F. et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 104, 170–178 (2009).
Daniels, A., van Bilsen, M., Goldschmeding, R., van der Vusse, G. J., & van Nieuwenhoven, F. A. Connective tissue growth factor and cardiac fibrosis. Acta Physiol. (Oxf.) 195, 321–338 (2009).
Pan, Z. et al. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway. Circulation 126, 840–850 (2012).
Heymans, S. et al. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation 128, 1420–1432 (2013).
Katare, R. et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 109, 894–906 (2011).
Akat, K. M. et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc. Natl Acad. Sci. USA 111, 11151–11156 (2014).
Marfella, R. et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: responders vs. non-responders. Eur. J. Heart Fail. 15, 1277–1288 (2013).
Morley-Smith, A. et al. 178 circulating microRNAs for predicting and monitoring response to mechanical circulatory support from a left ventricular assist device. Heart 100 (Suppl. 3), A100–A101 (2014).
Yang, K. C. et al. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129, 1009–1021 (2014).
Nattel, S., Harada, M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J. Am. Coll. Cardiol. 63, 2335–2345 (2014).
Lip, G. Y., Tse, H. F. & Lane, D. A. Atrial fibrillation. Lancet 379, 648–661 (2012).
Nishi, H. et al. Impact of microRNA expression in human atrial tissue in patients with atrial fibrillation undergoing cardiac surgery. PLoS ONE 8, e73397 (2013).
Cardin, S. et al. Role for microRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ. Arrhythm. Electrophysiol. 5, 1027–1035 (2012).
Adam, O. et al. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res. Cardiol. 107, 278 (2012).
Shan, H. et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc. Res. 83, 465–472 (2009).
Leite-Moreira, A. F. et al. ESC working group on myocardial function position paper: how to study the right ventricle in experimental models. Eur. J. Heart Fail. 16, 509–518 (2014).
Santangeli, P. et al. Fragmented and delayed electrograms within fibrofatty scar predict arrhythmic events in arrhythmogenic right ventricular cardiomyopathy: results from a prospective risk stratification study. Heart Rhythm 9, 1200–1206 (2012).
Vacchi-Suzzi, C. et al. Heart structure-specific transcriptomic atlas reveals conserved microRNA-mRNA interactions. PLoS ONE 8, e52442 (2013).
Drake, J. I. et al. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am. J. Respir. Cell. Mol. Biol. 45, 1239–1247 (2011).
Reddy, S. et al. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol. Genomics 44, 562–575 (2012).
Boon, R. A. et al. MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110 (2013).
Gupta, S. K., Piccoli, M. T., Thum, T. Non-coding RNAs in cardiovascular ageing. Ageing Res. Rev. http://dx.doi.org/10.1016/j.arr.2014.01.002.
Jazbutyte, V. et al. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr.) 35, 747–762 (2013).
Huang, Z. P. et al. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 112, 1234–1243 (2013).
van Almen, G. C. et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 10, 769–779 (2011).
Vassalli, G, Milano, G. & Moccetti, T. Role of mitogen-activated protein kinases in myocardial ischemia-reperfusion injury during heart transplantation. J. Transplant. 2012, 928954 (2012).
Lorenzen, J. M., Batkai, S. & Thum, T. Regulation of cardiac and renal ischemia-reperfusion injury by microRNAs. Free Radic. Biol. Med. 64, 78–84 (2013).
Zhou, L. et al. MicroRNA and mRNA signatures in ischemia reperfusion injury in heart transplantation. PLoS ONE 8, e79805 (2013).
Holmberg, F. E. et al. Conditioning techniques and ischemic reperfusion injury in relation to on-pump cardiac surgery. Scand. Cardiovasc. J. 48, 241–248 (2014).
Wang, E. et al. Circulating miRNAs reflect early myocardial injury and recovery after heart transplantation. J. Cardiothorac Surg. 8, 165 (2013).
Sagar, S., Liu, P. P. & Cooper, L. T. Jr. Myocarditis. Lancet 379, 738–747 (2012).
Corsten, M. F. et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 111, 415–425 (2012).
Cooper, L., Johnson, C., Burslem, F. & Martin, P. Wound healing and inflammation genes revealed by array analysis of macrophageless PU.1 null mice. Genome Biol. 6, R5 (2005).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat. Rev. Cardiol. 7, 564–575 (2010).
Horie, T. et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 87, 656–664 (2010).
Fu, J. et al. Let-7 g is involved in doxorubicin induced myocardial injury. Environ. Toxicol. Pharmacol. 33, 312–317 (2012).
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Nam, Y. J. et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl Acad. Sci. USA 110, 5588–5593 (2013).
Wada, R. et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc. Natl Acad. Sci. USA 110, 12667–12672 (2013).
Chen, J. X. et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 111, 50–55 (2012).
Jayawardena, T. M. et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465–1473 (2012).
Xue, Y. et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 152, 82–96 (2013).
Gupta, S. K., Bang, C. & Thum, T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Genet. 3, 484–488 (2010).
Jaguszewski, M. et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 35, 999–1006 (2014).
Lorenzen, J. M., Martino, F. & Thum, T. Detection and transport mechanisms of circulating microRNAs in neurological, cardiac and kidney diseases. Curr. Med. Chem. 20, 3623–3628 (2013).
Widera, C. et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell. Cardiol. 51, 872–875 (2011).
Roncarati, R. et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 63, 920–927 (2014).
Dawson, K. et al. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 127, 1466–1475 (2013).
Hergenreider, E. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 14, 249–256 (2012).
Sahoo, S. & Losordo, D. W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 114, 333–344 (2014).
Ounzain, S. et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehu180.
Wang, K. et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 114, 1377–1388 (2014).
Michalik, K. M. et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 114, 1389–1397 (2014).
Thum, T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol. Med. 4, 3–14 (2012).
Hinkel, R. et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 128, 1066–1075 (2013).
Quattrocelli, M. et al. Long-term miR-669a therapy alleviates chronic dilated cardiomyopathy in dystrophic mice. J. Am. Heart Assoc. 2, e000284 (2013).
Pihlmann, M. et al. Adeno-associated virus-delivered polycistronic microRNA-clusters for knockdown of vascular endothelial growth factor in vivo. J. Gene Med. 14, 328–338 (2012).
Wei, C. et al. NF-κB mediated miR-26a regulation in cardiac fibrosis. J. Cell. Physiol. 228, 1433–1442 (2013).
Bernardo, B. C. et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl Acad. Sci. USA 109, 17615–17620 (2012).
Beaumont, J. et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin. Sci. (Lond.) 126, 497–506 (2014).
da Costa Martins, P. A. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 12, 1220–1227 (2010).
Aurora, A. B. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca²+ overload and cell death. J. Clin. Invest. 122, 1222–1232 (2012).
Acknowledgements
T.T. acknowledges support from the European Commission-funded FIBROTARGET project, the Integrated Research and Treatment Centre Transplantation (grant number 01EO1302), and Fondation Leducq (Project MIRVAD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T.T. declares that he holds patents relating to the diagnostic and therapeutic use of ncRNAs in cardiovascular diseases.
Rights and permissions
About this article
Cite this article
Thum, T. Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol 11, 655–663 (2014). https://doi.org/10.1038/nrcardio.2014.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2014.125
This article is cited by
-
Noncoding RNAs regulating ferroptosis in cardiovascular diseases: novel roles and therapeutic strategies
Molecular and Cellular Biochemistry (2023)
-
MicroRNA-26a/b-5p promotes myocardial infarction-induced cell death by downregulating cytochrome c oxidase 5a
Experimental & Molecular Medicine (2021)
-
MicroRNA-99b-3p promotes angiotensin II-induced cardiac fibrosis in mice by targeting GSK-3β
Acta Pharmacologica Sinica (2021)
-
Circular RNAs as potential theranostics in the cardiac fibrosis
Heart Failure Reviews (2021)
-
Mitochondrial noncoding RNA-regulatory network in cardiovascular disease
Basic Research in Cardiology (2020)