Key Points
-
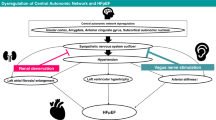
Heart failure with reduced ejection fraction (HFrEF) is initiated when an 'index event' causes the pumping capacity of the heart to be impaired
-
Reduced pumping capacity of the heart results in compensatory activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system, which together is referred to as 'neurohormonal activation'
-
Neurohormonal activation results in a series of coordinated responses that collectively work to restore cardiovascular homeostasis in the short term
-
Sustained neurohormonal activation drives the progression of HFrEF through the deleterious effects exerted on the circulation and the myocardium
-
Antagonism of neurohormonal systems forms the basis of modern therapy for HFrEF
Abstract
Heart failure with reduced ejection fraction (HFrEF) develops when cardiac output falls as a result of cardiac injury. The most well-recognized of the compensatory homeostatic responses to a fall in cardiac output are activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system (RAAS). In the short term, these 'neurohormonal' systems induce a number of changes in the heart, kidneys, and vasculature that are designed to maintain cardiovascular homeostasis. However, with chronic activation, these responses result in haemodynamic stress and exert deleterious effects on the heart and the circulation. Neurohormonal activation is now known to be one of the most important mechanisms underlying the progression of heart failure, and therapeutic antagonism of neurohormonal systems has become the cornerstone of contemporary pharmacotherapy for heart failure. In this Review, we discuss the effects of neurohormonal activation in HFrEF and highlight the mechanisms by which these systems contribute to disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mann, D. L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ. Res. 116, 1254–1268 (2015).
Rouleau, J. L. et al. Activation of neurohumoral systems following acute myocardial infarction. Am. J. Cardiol. 68, 80D–86D (1991).
Packer, M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 20, 248–254 (1992).
Piepoli, M. et al. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93, 940–952 (1996).
Giannoni, A. et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J. Am. Coll. Cardiol. 53, 1975–1980 (2009).
Ponikowski, P. P. et al. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104, 2324–2330 (2001).
Floras, J. S. & Ponikowski, P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur. Heart J. 36, 1974–1982 (2015).
Florea, V. G. & Cohn, J. N. The autonomic nervous system and heart failure. Circ. Res. 114, 1815–1826 (2014).
Sigurdsson, A. & Swedberg, K. The role of neurohormonal activation in chronic heart failure and postmyocardial infarction. Am. Heart J. 132, 229–234 (1996).
Cohn, J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
Dzau, V. J., Colucci, W. S., Hollenberg, N. K. & Williams, G. H. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 63, 645–651 (1981).
Francis, G. S. et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. Circulation 82, 1724–1729 (1990).
Mann, D. L., Kent, R. L., Parsons, B. & Cooper, G. I. V. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85, 790–804 (1992).
Adams, J. W. et al. Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc. Natl Acad. Sci. USA 95, 10140–10145 (1998).
Bisognano, J. D. et al. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J. Mol. Cell Cardiol. 32, 817–830 (2000).
Engelhardt, S., Hein, L., Wiesmann, F. & Lohse, M. J. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl Acad. Sci. USA 96, 7059–7064 (1999).
Bozkurt, B. et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97, 1382–1391 (1998).
Teerlink, J. R., Pfeffer, J. M. & Pfeffer, M. A. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ. Res. 75, 105–113 (1994).
Cohn, J. N. et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N. Engl. J. Med. 325, 303–310 (1991).
Packer, M. et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 334, 1350–1355 (1996).
Pitt, B. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 341, 709–717 (1999).
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N. Engl. J. Med. 325, 293 (1991).
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353, 2001–2007 (1999).
Bristow, M. R. et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 94, 2807–2816 (1996).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 15, e147–e239 (2013).
Notarius, C. F., Millar, P. J. & Floras, J. S. Muscle sympathetic activity in resting and exercising humans with and without heart failure. Appl. Physiol. Nutr. Metab. 40, 1107–1115 (2015).
Weinberger, M. H., Aoi, W. & Henry, D. P. Direct effect of beta-adrenergic stimulation on renin release by the rat kidney slice in vitro. Circ. Res. 37, 318–324 (1975).
Bekheirnia, M. R. & Schrier, R. W. Pathophysiology of water and sodium retention: edematous states with normal kidney function. Curr. Opin. Pharmacol. 6, 202–207 (2006).
McCollum, L. T., Gallagher, P. E. & Ann Tallant, E. Angiotensin-(1–7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am. J. Physiol. Heart Circ. Physiol. 302, H801–H810 (2012).
Wamberg, C., Plovsing, R. R., Sandgaard, N. C. & Bie, P. Effects of different angiotensins during acute, double blockade of the renin system in conscious dogs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R971–980 (2003).
Huang, B. S. et al. Inhibition of brain angiotensin III attenuates sympathetic hyperactivity and cardiac dysfunction in rats post-myocardial infarction. Cardiovasc. Res. 97, 424–431 (2013).
Esteban, V. et al. Angiotensin IV activates the nuclear transcription factor-kappaB and related proinflammatory genes in vascular smooth muscle cells. Circ. Res. 96, 965–973 (2005).
Bomback, A. S. & Klemmer, P. J. The incidence and implications of aldosterone breakthrough. Nat. Clin. Pract. Nephrol. 3, 486–492 (2007).
Schrier, R. W. Aldosterone 'escape' versus 'breakthrough'. Nat. Rev. Nephrol. 6, 61 (2010).
Pitt, B., Remme, W. & Zannad, F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 348, 1309–1321 (2003).
Braunwald, E. The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure. J. Am. Coll. Cardiol. 65, 1029–1041 (2015).
Schrier, R. W. & Abraham, W. T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 341, 577–585 (1999).
Clerico, A., Recchia, F. A., Passino, C. & Emdin, M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 290, H17–H29 (2006).
Volpe, M., Carnovali, M. & Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin. Sci. (Lond.) 130, 57–77 (2016).
McMurray, J. J. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 317, 993–1004 (2014).
Shah, A. M. & Mann, D. L. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 378, 704–712 (2011).
Mann, D. L. & Bristow, M. R. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111, 2837–2849 (2005).
Toischer, K. et al. Differential cardiac remodeling in preload versus afterload. Circulation 122, 993–1003 (2010).
van Berlo, J. H., Maillet, M. & Molkentin, J. D. Signaling effectors underlying pathologic growth and remodeling of the heart. J. Clin. Invest. 123, 37–45 (2013).
Lowes, B. D. et al. Changes in gene expression in the intact human heart. Downregulation of alpha-Myosin heavy chain in hypertrophied, failing ventricular myocardium. J. Clin. Invest. 100, 2315–2324 (1997).
Kostin, S., Hein, S., Arnon, E., Scholz, D. & Schaper, J. The cytoskeleton and related proteins in the human failing heart. Heart Fail. Rev. 5, 271–280 (2000).
Hein, S., Kostin, S., Heling, A., Maeno, Y. & Schaper, J. The role of the cytoskeleton in heart failure. Cardiovasc. Res. 45, 273–278 (2000).
Rockman, H. A., Koch, W. J. & Lefkowitz, R. J. Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212 (2002).
Lymperopoulos, A., Rengo, G. & Koch, W. J. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ. Res. 113, 739–753 (2013).
Feldman, D. S., Carnes, C. A., Abraham, W. T. & Bristow, M. R. Mechanisms of disease: beta-adrenergic receptors—alterations in signal transduction and pharmacogenomics in heart failure. Nat. Clin. Pract. Cardiovasc. Med. 2, 475–483 (2005).
Port, J. D. & Bristow, M. R. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J. Mol. Cell Cardiol. 33, 887–905 (2001).
Bristow, M. R. et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 307, 205–211 (1982).
Bristow, M. R. et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ. Res. 59, 297–309 (1986).
Reiter, E. & Lefkowitz, R. J. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165 (2006).
Ungerer, M., Bohm, M., Elce, J. S., Erdmann, E. & Lohse, M. J. Altered expression of beta-adrenergic receptor kinase and beta1-adrenergic receptors in the failing human heart. Circulation 87, 454–463 (1993).
Iaccarino, G., Tomhave, E. D., Lefkowitz, R. J. & Koch, W. J. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation 98, 1783–1789 (1998).
Rockman, H. A. et al. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA 95, 7000–7005 (1998).
Rengo, G. et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation 119, 89–98 (2009).
Raake, P. W. et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur. Heart J. 34, 1437–1447 (2013).
Harding, V. B., Jones, L. R., Lefkowitz, R. J., Koch, W. J. & Rockman, H. A. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc. Natl Acad. Sci. USA 98, 5809–5814 (2001).
Rajabi, M., Kassiotis, C., Razeghi, P. & Taegtmeyer, H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 12, 331–343 (2007).
Lowes, B. D. et al. Myocardial gene expression in dilated cardiomyopathy treated with beta- blocking agents. N. Engl. J. Med. 346, 1357–1365 (2002).
Brooks, W. W. et al. Captopril modifies gene expression in hypertrophied and failing hearts of aged spontaneously hypertensive rats. Hypertension 30, 1362–1368 (1997).
Wang, J., Guo, X. & Dhalla, N. S. Modification of myosin protein and gene expression in failing hearts due to myocardial infarction by enalapril or losartan. Biochim. Biophys. Acta 1690, 177–184 (2004).
Marks, A. R. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J. Clin. Invest. 123, 46–52 (2013).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
Arai, M., Alpert, N. R., MacLennan, D. H., Barton, P. & Periasamy, M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. Circ. Res. 72, 463–469 (1993).
Hasenfuss, G. et al. Relation between myocardial function and expression of sarcoplasmic reticulum ca2+-ATPase in failing and nonfailing human myocardium. Circ. Res. 75, 434–442 (1994).
Reiken, S. et al. Beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104, 2843–2848 (2001).
Reiken, S. et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107, 2459–2466 (2003).
Mann, D. L. Left ventricular size and shape: determinants of mechanical signal transduction pathways. Heart Fail. Rev. 10, 95–100 (2005).
Guerra, S. et al. Myocyte death in the failing human heart is gender dependent. Circ. Res. 85, 856–866 (1999).
Kostin, S. et al. Myocytes die by multiple mechanisms in failing human hearts. Circ. Res. 92, 715–724 (2003).
Whelan, R. S., Kaplinskiy, V. & Kitsis, R. N. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 72, 19–44 (2010).
Kroemer, G. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 (2009).
Tan, L. B., Jalil, J. E., Pick, R., Janicki, J. S. & Weber, K. T. Cardiac myocyte necrosis induced by angiotensin II. Circ. Res. 69, 1185–1195 (1991).
Todd, G. L., Baroldi, G., Pieper, G. M., Clayton, F. C. & Eliot, R. S. Experimental catecholamine-induced myocardial ncrosis I. Morphology, quantification and regional distribution of acute contraction band lesions. J. Mol. Cell. Cardiol. 17, 317–338 (1985).
Zhang, W. et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J. Am. Heart Assoc. 4, e001993 (2015).
Epelman, S., Liu, P. P. & Mann, D. L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 15, 117–129 (2015).
Olivetti, G. et al. Apoptosis in the failing human heart. N. Engl. J. Med. 336, 1131–1141 (1997).
Saraste, A. et al. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur. J. Clin. Invest. 29, 380–386 (1999).
Wencker, D. et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 111, 1497–1504 (2003).
Communal, C., Singh, K., Sawyer, D. B. & Colucci, W. S. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation 100, 2210–2212 (1999).
Haudek, S. B., Taffet, G. E., Schneider, M. D. & Mann, D. L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Invest. 117, 2692–2701 (2007).
Kajstura, J. et al. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J. Mol. Cell Cardiol. 29, 859–870 (1997).
Kroemer, G., Marino, G. & Levine, B. Autophagy and the integrated stress response. Mol. Cell 40, 280–293 (2010).
Lavandero, S., Chiong, M., Rothermel, B. A. & Hill, J. A. Autophagy in cardiovascular biology. J. Clin. Invest. 125, 55–64 (2015).
Ma, X. et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125, 3170–3181 (2012).
Kong, P., Christia, P. & Frangogiannis, N. G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71, 549–574 (2014).
Davis, J. & Molkentin, J. D. Myofibroblasts: trust your heart and let fate decide. J. Mol. Cell Cardiol. 70, 9–18 (2014).
Hartupee, J. & Mann, D. L. Role of inflammatory cells in fibroblast activation. J. Mol. Cell Cardiol 93, 143–148 (2016).
Schorb, W. et al. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ. Res. 72, 1245–1254 (1993).
Sadoshima, J. I. & Izumo, S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 73, 413–423 (1993).
Brilla, C. G., Funck, R. C. & Rupp, H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102, 1388–1393 (2000).
Izawa, H. et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation 112, 2940–2945 (2005).
Zannad, F., Alla, F., Dousset, B., Perez, A. & Pitt, B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 102, 2700–2706 (2000).
Li, Y. Y., Feldman, A. M., Sun, Y. & McTiernan, C. F. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation 98, 1728–1734 (1998).
Creemers, E. E. et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 284, H364–H371 (2003).
Ducharme, A. et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 106, 55–62 (2000).
Kim, H. E. et al. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J. Clin. Invest. 106, 857–866 (2000).
Peterson, J. T. et al. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 103, 2303–2309 (2001).
Koitabashi, N. & Kass, D. A. Reverse remodeling in heart failure—mechanisms and therapeutic opportunities. Nat. Rev. Cardiol. 9, 147–157 (2012).
Mann, D. L., Barger, P. M. & Burkhoff, D. Myocardial recovery: myth, magic or molecular target? J. Am. Coll. Cardiol. 60, 2465–2472 (2012).
Acknowledgements
The authors' research is supported by research funds from the NIH (R01 HL58081, RO1 111094, T32 HL007081).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
D.L.M. is a consultant to Novartis and is on the steering committee for the PARADISE trial. J.H. declares no competing interests.
Rights and permissions
About this article
Cite this article
Hartupee, J., Mann, D. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 14, 30–38 (2017). https://doi.org/10.1038/nrcardio.2016.163
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.163
This article is cited by
-
Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice
Journal of Neuroinflammation (2024)
-
Renal sympathetic denervation improves pressure-natriuresis relationship in cardiorenal syndrome: insight from studies with Ren-2 transgenic hypertensive rats with volume overload induced using aorto-caval fistula
Hypertension Research (2024)
-
Kinin-kallikrein system: New perspectives in heart failure
Heart Failure Reviews (2024)
-
The prognostic role of urea-to-creatinine ratio in patients with acute heart failure syndrome: a case–control study
The Egyptian Heart Journal (2023)
-
A kidney-brain neural circuit drives progressive kidney damage and heart failure
Signal Transduction and Targeted Therapy (2023)