Key Points
-
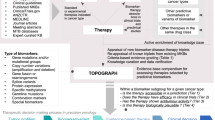
Predictive biomarkers are essential tools with regard to personalized medicine and health economics, and are crucial to improve the success rate of new therapies
-
Implementation of biomarkers into clinical practice presents biological, clinical and logistical challenges, in particular, relating to standardization across multiple countries and clinical practices
-
During biomarker development, robust laboratory methodology is necessary at all analytical phases, from pre-analytical (sample definition, handling and processing) to analytical (data and quality-control recording) and post-analytical (data reporting and interpretation)
-
A series of recommendations can be made to increase biomarker reliability and facilitate development of predictive biomarkers that can ultimately be used to provide benefit for patients with cancer
Abstract
Predictive biomarkers are becoming increasingly important tools in drug development and clinical research. The importance of using both guidelines for specimen acquisition and analytical methods for biomarker measurements that are standardized has become recognized widely as an important issue, which must be addressed in order to provide high-quality, validated assays. Herein, we review the major challenges in biomarker validation processes, including pre-analytical (sample-related), analytical, and post-analytical (data-related) aspects of assay development. Recommendations for improving biomarker assay development and method validation are proposed to facilitate the use of predictive biomarkers in clinical trials and the practice of oncology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Giantonio, B. J. et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 25, 1539–1544 (2007).
Saltz, L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 26, 2013–2019 (2008).
Taube, S. E. et al. A perspective on challenges and issues in biomarker development and drug and biomarker codevelopment. J. Natl Cancer Inst. 101, 1453–1463 (2009).
DiMasi, J. A., Feldman, L., Seckler, A. & Wilson, A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin. Pharmacol. Ther. 87, 272–277 (2010).
Arrowsmith, J. Trial watch: phase II failures: 2008–2010. Nat. Rev. Drug Discov. 10, 328–329 (2011).
Arrowsmith, J. Trial watch: phase III and submission failures: 2007–2010. Nat. Rev. Drug Discov. 10, 87 (2011).
Huriez, A. Personalized medicine, introduction, business impact on the healthcare sector and regulatory aspects [online], (2013).
Cummings, J., Raynaud, F., Jones, L., Sugar, R. & Dive, C. Fit-for-purpose biomarker method validation for application in clinical trials of anticancer drugs. Br. J. Cancer 103, 1313–1317 (2010).
Garcia, V. M., Cassier, P. A. & de Bono, J. Parallel anticancer drug development and molecular stratification to qualify predictive biomarkers: dealing with obstacles hindering progress. Cancer Discov. 1, 207–212 (2011).
Lee, J. W. et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm. Res. 23, 312–328 (2006).
Walker, R. A. Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment, I. Histopathology 49, 406–410 (2006).
Boenisch, T. Can a more selective application of antigen retrieval facilitate standardization in immunohistochemistry? Appl. Immunohistochem. Mol. Morphol. 12, 172–176 (2004).
Goldstein, N. S., Hewitt, S. M., Taylor, C. R., Yaziji, H. & Hicks, D. G. Recommendations for improved standardization of immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 15, 124–133 (2007).
Office of Biorepositories and Biospecimen Research. NCI Best Practices for Biospecimen Resources [online], (2014).
Aktas, B. et al. Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecol. Oncol. 122, 356–360 (2011).
Amir, E. et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J. Clin. Oncol. 30, 587–592 (2012).
Aoyama, K., Kamio, T., Nishikawa, T. & Kameoka, S. A comparison of HER2/neu gene amplification and its protein overexpression between primary breast cancer and metastatic lymph nodes. Jpn J. Clin. Oncol. 40, 613–619 (2010).
Botteri, E. et al. Biopsy of liver metastasis for women with breast cancer: impact on survival. Breast 21, 284–288 (2012).
Cardoso, F. et al. Evaluation of HER2, p53, bcl-2, topoisomerase II-α, heat shock proteins 27 and 70 in primary breast cancer and metastatic ipsilateral axillary lymph nodes. Ann. Oncol. 12, 615–620 (2001).
Chang, H. J. et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J. Clin. Oncol. 41, 593–599 (2011).
Curigliano, G. et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann. Oncol. 22, 2227–2233 (2011).
Dieci, M. V. et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann. Oncol. 24, 101–108 (2013).
Duchnowska, R. et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 14, R119 (2012).
Fabi, A. et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin. Cancer Res. 17, 2055–2064 (2011).
Fehm, T. et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 11, R59 (2009).
Fuchs, I. B. et al. Epidermal growth factor receptor changes during breast cancer metastasis. Anticancer Res. 26, 4397–4401 (2006).
Gancberg, D. et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann. Oncol. 13, 1036–1043 (2002).
Gong, Y., Booser, D. J. & Sneige, N. Comparison of HER-2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer 103, 1763–1769 (2005).
Guarneri, V. et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist 13, 838–844 (2008).
Jensen, J. D., Knoop, A., Ewertz, M. & Laenkholm, A. V. ER, HER2, and TOP2A expression in primary tumor, synchronous axillary nodes, and asynchronous metastases in breast cancer. Breast Cancer Res. Treat. 132, 511–521 (2012).
Lear-Kaul, K. C., Yoon, H. R., Kleinschmidt-DeMasters, B. K., McGavran, L. & Singh, M. HER-2/neu status in breast cancer metastases to the central nervous system. Arch. Pathol. Lab. Med. 127, 1451–1457 (2003).
Lindström, L. S. et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 30, 2601–2608 (2012).
Lower, E. E., Glass, E., Blau, R. & Harman, S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res. Treat. 113, 301–306 (2009).
Macfarlane, R. et al. Molecular alterations between the primary breast cancer and the subsequent locoregional/metastatic tumor. Oncologist 17, 172–178 (2012).
Montagna, E. et al. Breast cancer subtypes and outcome after local and regional relapse. Ann. Oncol. 23, 324–331 (2012).
Niikura, N. et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 30, 593–599 (2012).
Regitnig, P., Schippinger, W., Lindbauer, M., Samonigg, H. & Lax, S. F. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J. Pathol. 203, 918–926 (2004).
Santinelli, A., Pisa, E., Stramazzotti, D. & Fabris, G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int. J. Cancer 122, 999–1004 (2008).
Shimizu, C. et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J. Surg. Oncol. 73, 17–20 (2000).
Simon, R. et al. Patterns of HER-2/neu amplification and overexpression in primary and metastatic breast cancer. J. Natl Cancer Inst. 93, 1141–1146 (2001).
Simmons, C. et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann. Oncol. 20, 1499–1504 (2009).
Thompson, A. M. et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 12, R92 (2010).
Vincent-Salomon, A. et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer 94, 2169–2173 (2002).
Wilking, U. et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res. Treat. 125, 553–561 (2011).
Xiao, C., Gong, Y., Han, E. Y., Gonzalez-Angulo, A. M. & Sneige, N. Stability of HER2-positive status in breast carcinoma: a comparison between primary and paired metastatic tumors with regard to the possible impact of intervening trastuzumab treatment. Ann. Oncol. 22, 1547–1553 (2011).
Zidan, J. et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br. J. Cancer 93, 552–556 (2005).
Albanese, I. et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem. Biophys. Res. Commun. 325, 784–791 (2004).
Al-Mulla, F. et al. Heterogeneity of mutant versus wild-type Ki-ras in primary and metastatic colorectal carcinomas, and association of codon-12 valine with early mortality. J. Pathol. 185, 130–138 (1998).
Artale, S. et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J. Clin. Oncol. 26, 4217–4219 (2008).
Baldus, S. E. et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin. Cancer Res. 16, 790–799 (2010).
Cejas, P. et al. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS ONE 4, e8199 (2009).
Cejas, P. et al. Analysis of the concordance in the EGFR pathway status between primary tumors and related metastases of colorectal cancer patients: implications for cancer therapy. Curr. Cancer Drug Targets 12, 124–131 (2012).
Etienne-Grimaldi, M. C. et al. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin. Cancer Res. 14, 4830–4835 (2008).
Finkelstein, S. D., Sayegh, R., Christensen, S. & Swalsky, P. A. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer 71, 3827–3838 (1993).
Garm Spindler, K. L. et al. The importance of KRAS mutations and EGF61A>G polymorphism to the effect of cetuximab and irinotecan in metastatic colorectal cancer. Ann. Oncol. 20, 879–884 (2009).
Italiano, A. et al. KRAS and BRAF mutational status in primary colorectal tumors and related metastatic sites: biological and clinical implications. Ann. Surg. Oncol. 17, 1429–1434 (2010).
Knijn, N. et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br. J. Cancer 104, 1020–1026 (2011).
Losi, L., Benhattar, J. & Costa, J. Stability of K-ras mutations throughout the natural history of human colorectal cancer. Eur. J. Cancer 28A, 1115–1120 (1992).
Loupakis, F. et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J. Clin. Oncol. 27, 2622–2629 (2009).
Mariani, P. et al. Concordant analysis of KRAS status in primary colon carcinoma and matched metastasis. Anticancer Res. 30, 4229–4235 (2010).
Melucci, E. et al. Relationship between K-Ras mutational status and EGFR expression evaluated using Allred score in primary and metastatic colorectal cancer [abstract]. J. Clin. Oncol. 28 (Suppl.), a3568 (2010).
Molinari, F. et al. Differing deregulation of EGFR and downstream proteins in primary colorectal cancer and related metastatic sites may be clinically relevant. Br. J. Cancer 100, 1087–1094 (2009).
Mostert, B. et al. KRAS and BRAF mutation status in circulating colorectal tumor cells and their correlation with primary and metastatic tumor tissue. Int. J. Cancer 133, 130–141 (2013).
Oliveira, C. et al. KRAS and BRAF oncogenic mutations in MSS colorectal carcinoma progression. Oncogene 26, 158–163 (2007).
Oltedal, S. et al. Heterogeneous distribution of K-ras mutations in primary colon carcinomas: implications for EGFR-directed therapy. Int. J. Colorectal Dis. 26, 1271–1277 (2011).
Oudejans, J. J., Slebos, R. J., Zoetmulder, F. A., Mooi, W. J. & Rodenhuis, S. Differential activation of ras genes by point mutation in human colon cancer with metastases to either lung or liver. Int. J. Cancer 49, 875–879 (1991).
Park, J. H. et al. Analysis of KRAS, BRAF, PTEN, IGF1R, EGFR intron 1 CA status in both primary tumors and paired metastases in determining benefit from cetuximab therapy in colon cancer. Cancer Chemother. Pharmacol. 68, 1045–1055 (2011).
Perrone, F. et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann. Oncol. 20, 84–90 (2009).
Santini, D. et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist 13, 1270–1275 (2008).
Schimanski, C. C., Linnemann, U. & Berger, M. R. Sensitive detection of K-ras mutations augments diagnosis of colorectal cancer metastases in the liver. Cancer Res. 59, 5169–5175 (1999).
Thebo, J. S., Senagore, A. J., Reinhold, D. S. & Stapleton, S. R. Molecular staging of colorectal cancer: K-ras mutation analysis of lymph nodes upstages Dukes B patients. Dis. Colon Rectum 43, 155–159; discussion 159–162 (2000).
Watanabe, T. et al. Heterogeneity of KRAS status may explain the subset of discordant KRAS status between primary and metastatic colorectal cancer. Dis. Colon Rectum 54, 1170–1178 (2011).
Zauber, P., Sabbath-Solitare, M., Marotta, S. P. & Bishop, D. T. Molecular changes in the Ki-ras and APC genes in primary colorectal carcinoma and synchronous metastases compared with the findings in accompanying adenomas. Mol. Pathol. 56, 137–140 (2003).
Santini, D. et al. High concordance of BRAF status between primary colorectal tumours and related metastatic sites: implications for clinical practice. Ann. Oncol. 21, 1565 (2010).
Jancik, S. et al. A comparison of direct sequencing, pyrosequencing, high resolution melting analysis, TheraScreen DxS, and the K-ras StripAssay for detecting KRAS mutations in non small cell lung carcinomas. J. Exp. Clin. Cancer Res. 31, 79 (2012).
Davidson, C. J. et al. Improving the limit of detection for Sanger sequencing: a comparison of methodologies for KRAS variant detection. Biotechniques 53, 182–188 (2012).
Ihle, M. A. et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer 14, 13 (2014).
Suchy, B., Zietz, C. & Rabes, H. M. K-ras point mutations in human colorectal carcinomas: relation to aneuploidy and metastasis. Int. J. Cancer 52, 30–33 (1992).
Vignot, S. et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J. Clin. Oncol. 31, 2167–2172 (2013).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Schwarzenbach, H., Hoon, D. S. & Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 11, 426–437 (2011).
Camp, E. R. et al. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin. Cancer Res. 11, 397–405 (2005).
Laurent-Puig, P. et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J. Clin. Oncol. 27, 5924–5930 (2009).
De Roock, W. et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11, 753–762 (2010).
Douillard, J. Y. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 369, 1023–1034 (2013).
Bredel, M. et al. Amplification of whole tumor genomes and gene-by-gene mapping of genomic aberrations from limited sources of fresh-frozen and paraffin-embedded DNA. J. Mol. Diagn. 7, 171–182 (2005).
Karapetis, C. S. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Buyse, M. et al. Integrating biomarkers in clinical trials. Expert Rev. Mol. Diagn. 11, 171–182 (2011).
Buyse, M., Sargent, D. J., Grothey, A., Matheson, A. & de Gramont, A. Biomarkers and surrogate end points—the challenge of statistical validation. Nat. Rev. Clin. Oncol. 7, 309–317 (2010).
Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labels [online], (2014).
Libeer, J. C. & Ehrmeyer, S. ISO 15189: a worldwide standard for medical laboratories. Point of Care 3, 5–7 (2004).
International Society for Biological and Environmental Repositories. ISBER Best Practices for Repositories [online], (2014).
College of American Pathologists. Accreditation and Laboratory Improvement [online], (2014).
National Cancer Institute. NCI Biospecimen Research Database [online], (2014).
Mitchell, B. L., Yasui, Y., Li, C. I., Fitzpatrick, A. L. & Lampe, P. D. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform. 1, 98–104 (2005).
Siddiqui, S. & Rimm, D. L. Pre-analytic variables and phospho-specific antibodies: the Achilles heel of immunohistochemistry. Breast Cancer Res. 12, 113 (2010).
Johnsen, I. K. et al. Evaluation of a standardized protocol for processing adrenal tumor samples: preparation for a European adrenal tumor bank. Horm. Metab. Res. 42, 93–101 (2010).
Chung, J. Y. et al. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J. Histochem. Cytochem. 56, 1033–1042 (2008).
Medeiros, F., Rigl, C. T., Anderson, G. G., Becker, S. H. & Halling, K. C. Tissue handling for genome-wide expression analysis: a review of the issues, evidence, and opportunities. Arch. Pathol. Lab. Med. 131, 1805–1816 (2007).
Douglas, M. P. & Rogers, S. O. DNA damage caused by common cytological fixatives. Mutat. Res. 401, 77–88 (1998).
Williams, C. et al. A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am. J. Pathol. 155, 1467–1471 (1999).
Wong, S. Q. et al. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genomics 7, 23 (2014).
Guo, H. et al. An efficient procedure for protein extraction from formalin-fixed, paraffin-embedded tissues for reverse phase protein arrays. Proteome Sci. 10, 56 (2012).
Sprung, R. W. Jr et al. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol. Cell. Proteomics 8, 1988–1998 (2009).
Fedorowicz, G., Guerrero, S., Wu, T. D. & Modrusan, Z. Microarray analysis of RNA extracted from formalin-fixed, paraffin-embedded and matched fresh-frozen ovarian adenocarcinomas. BMC Med. Genomics 2, 23 (2009).
Kalmar, A. et al. Gene expression analysis of normal and colorectal cancer tissue samples from fresh frozen and matched formalin-fixed, paraffin-embedded (FFPE) specimens after manual and automated RNA isolation. Methods 59, S16–S19 (2013).
Xie, R. et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J. Histochem. Cytochem. 59, 356–365 (2011).
Liu, N. W. et al. Impact of ischemia and procurement conditions on gene expression in renal cell carcinoma. Clin. Cancer Res. 19, 42–49 (2013).
De Marzo, A. M., Fine, S. & Trock, B. J. Impact of Pre-Analytic Variation on Tissue Analysis: Issues & Practical Applications. Presented at the 2008 Biospecimen Research Network Symposium (2008).
Blons, H. & Laurent-Puig, P. Technical considerations for KRAS testing in colorectal cancer. The biologist's point of view [French]. Bull. Cancer 96 (Suppl.), S47–S56 (2009).
Press, M. F. et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin. Cancer Res. 11, 6598–6607 (2005).
Perez, E. A. et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J. Clin. Oncol. 24, 3032–3038 (2006).
Fitzgibbons, P. L., Murphy, D. A., Dorfman, D. M., Roche, P. C. & Tubbs, R. R. Interlaboratory comparison of immunohistochemical testing for HER2: results of the 2004 and 2005 College of American Pathologists HER2 Immunohistochemistry Tissue Microarray Survey. Arch. Pathol. Lab. Med. 130, 1440–1445 (2006).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 25, 118–145 (2007).
Taylor, C. R. & Levenson, R. M. Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment II. Histopathology 49, 411–424 (2006).
Pauletti, G. et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J. Clin. Oncol. 18, 3651–3664 (2000).
Dowsett, M. et al. Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod. Pathol. 20, 584–591 (2007).
Lievre, A. et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 26, 374–379 (2008).
Hindson, B. J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604–8610 (2011).
Pekin, D. et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 11, 2156–2166 (2011).
Andre, T. et al. Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann. Oncol. 24, 412–419 (2013).
van't Veer, L. J. & Bernards, R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature 452, 564–570 (2008).
Paik, S., Kim, C. & Wolmark, N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N. Engl. J. Med. 358, 1409–1411 (2008).
Fehrenbacher, L. et al. NSABP B-47: a randomized phase III trial of adjuvant therapy comparing chemotherapy alone to chemotherapy plus trastuzumab in women with node-positive or high-risk node-negative HER2-low invasive breast cancer [abstract]. J. Clin. Oncol. 31 (Suppl.), TPS1139 (2013).
Teixidó, C., Karachaliou, N., Peg, V., Gimenez-Capitan, A. & Rosell, R. Concordance of IHC, FISH and RT-PCR for EML4–ALK rearrangements. Transl. Lung Cancer Res. 3, 70–74 (2014).
Angulo, B. et al. A comparison of EGFR mutation testing methods in lung carcinoma: direct sequencing, real-time PCR and immunohistochemistry. PLoS ONE 7, e43842 (2012).
Personeni, N. et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J. Hepatol. 57, 101–107 (2012).
Haynes, B. P. et al. Expression of key oestrogen-regulated genes differs substantially across the menstrual cycle in oestrogen receptor-positive primary breast cancer. Breast Cancer Res. Treat. 138, 157–165 (2013).
von Minckwitz, G. et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin. Cancer Res. 19, 4521–4531 (2013).
Chandarlapaty, S. et al. Frequent mutational activation of the PI3K–AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res. 18, 6784–6791 (2012).
Lorgis, V. et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast 20, 284–287 (2011).
Arnedos, M. et al. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann. Oncol. 20, 1948–1952 (2009).
Stalhammar, G., Rosin, G., Fredriksson, I., Bergh, J. & Hartman, J. Low concordance of biomarkers in histopathological and cytological material from breast cancer. Histopathology 64, 971–980 (2014).
Engel, K. B. & Moore, H. M. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab. Med. 135, 537–543 (2011).
Khoury, T. et al. Delay to formalin fixation effect on breast biomarkers. Mod. Pathol. 22, 1457–1467 (2009).
Marchetti, A., Felicioni, L. & Buttitta, F. Assessing EGFR mutations. N. Engl. J. Med. 354, 526–528 (2006).
Punnoose, E. A. et al. Molecular biomarkers analyses using circulating tumor cells. PLoS ONE 5, e12517 (2010).
Voros, A., Csorgo, E., Nyari, T. & Cserni, G. An intra- and interobserver reproducibility analysis of the Ki-67 proliferation marker assessment on core biopsies of breast cancer patients and its potential clinical implications. Pathobiology 80, 111–118 (2013).
Ronot, M. et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist 19, 394–402 (2014).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Cobleigh, M. A. et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 17, 2639–2648 (1999).
Heinrich, M. C. et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 21, 4342–4349 (2003).
Blanke, C. D. et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J. Clin. Oncol. 26, 620–625 (2008).
Kang, M. J. et al. Biweekly cetuximab plus irinotecan as second-line chemotherapy for patients with irinotecan-refractory and KRAS wild-type metastatic colorectal cancer according to epidermal growth factor receptor expression status. Invest. New Drugs 30, 1607–1613 (2012).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Schwartzberg, L. S. et al. Analysis of KRAS/NRAS mutations in PEAK: a randomized phase II study of FOLFOX6 plus panitumumab (pmab) or bevacizumab (bev) as first-line treatment (tx) for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) [abstract]. J. Clin. Oncol. 31 (Suppl.), a3631 (2013).
Cameron, D. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 112, 533–543 (2008).
Martin, M. et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur. J. Cancer 49, 3763–3772 (2013).
McArthur, G. A. et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J. Clin. Oncol. 23, 866–873 (2005).
Kerob, D. et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin. Cancer Res. 16, 3288–3295 (2010).
Hirsch, F. R. et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J. Clin. Oncol. 24, 5034–5042 (2006).
Sutani, A. et al. Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br. J. Cancer 95, 1483–1489 (2006).
Villanueva, C. et al. Phase II study assessing lapatinib added to letrozole in patients with progressive disease under aromatase inhibitor in metastatic breast cancer-Study BES 06. Target. Oncol. 8, 137–143 (2013).
Johnston, S. et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 27, 5538–5546 (2009).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Malik, S. M. et al. U. S. Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin. Cancer Res. 20, 2029–2034 (2014).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
McArthur, G. A. et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 15, 323–332 (2014).
Bokemeyer, C. et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 48, 1466–1475 (2012).
Tveit, K. M. et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J. Clin. Oncol. 30, 1755–1762 (2012).
Baselga, J. et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J. Clin. Oncol. 28, 1138–1144 (2010).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 14, 461–471 (2013).
Hurvitz, S. A. et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 31, 1157–1163 (2013).
Burris, H. A. 3rd et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 (2011).
Sequist, L. V. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31, 3327–3334 (2013).
Wu, Y. L. et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 15, 213–222 (2014).
Shaw, A. T. et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 370, 1189–1197 (2014).
Vansteenkiste, J. F. Ceritinib for treatment of ALK-rearranged advanced non-small-cell lung cancer. Future Oncol. 10, 1925–1939 (2014).
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12, 735–742 (2011).
Kim, S. T. et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer 75, 82–88 (2012).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284 (2013).
Flaherty, K. T. et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 367, 1694–1703 (2012).
Kim, K. B. et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 31, 482–489 (2013).
Long, G. V. et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 1087–1095 (2012).
Sosman, J. A. et al. BRAF inhibitor (BRAFi) dabrafenib in combination with the MEK1/2 inhibitor (MEKi) trametinib in BRAFi-naive and BRAFi-resistant patients (pts) with BRAF mutation-positive metastatic melanoma (MM) [abstract]. J. Clin. Oncol. 31 (Suppl.), a9005 (2013).
European Medicines Agency (EMA). European public assessment reports [online], (2014).
Lee, J. W. et al. Method validation and measurement of biomarkers in nonclinical and clinical samples in drug development: a conference report. Pharm. Res. 22, 499–511 (2005).
Acknowledgements
The work of the authors has been supported by the Aide et Recherche en Cancérologie Digestive (ARCAD) foundation, a not-for-profit organization, and editorial assistance was provided by M. Benetkiewicz.
Author information
Authors and Affiliations
Contributions
Armand de Gramont, S.W., Aimery de Gramont and S.R.H. researched the data from the article; Armand de Gramont, L.M.E., J.R., J.T. Aimery de Gramont and S.R.H. contributed substantially to writing the article; and all authors contributed to discussion of content and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
de Gramont, A., Watson, S., Ellis, L. et al. Pragmatic issues in biomarker evaluation for targeted therapies in cancer. Nat Rev Clin Oncol 12, 197–212 (2015). https://doi.org/10.1038/nrclinonc.2014.202
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.202
This article is cited by
-
Isothermal calorimetry calscreener in the metabolism gauge of human malignant neoplastic cells: a burgeoning nexus in cancer biochemical metrology and diagnostics
Bulletin of the National Research Centre (2023)
-
Serum biomarker-based early detection of pancreatic ductal adenocarcinomas with ensemble learning
Communications Medicine (2023)
-
Applicability of PD-L1 tests to tailor triple-negative breast cancer treatment in Brazil
Surgical and Experimental Pathology (2021)
-
RAB5A expression is a predictive biomarker for trastuzumab emtansine in breast cancer
Nature Communications (2021)
-
Toward precision immunotherapy using multiplex immunohistochemistry and in silico methods to define the tumor immune microenvironment
Cancer Immunology, Immunotherapy (2021)